Epitope-based vaccines with the Anaplasma marginale MSP1a functional motif induce a balanced humoral and cellular immune response in mice
- PMID: 23579784
- PMCID: PMC3620323
- DOI: 10.1371/journal.pone.0060311
Epitope-based vaccines with the Anaplasma marginale MSP1a functional motif induce a balanced humoral and cellular immune response in mice
Abstract
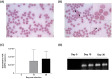
Bovine anaplasmosis is a hemoparasitic disease that causes considerable economic loss to the dairy and beef industries. Cattle immunized with the Anaplasma marginale MSP1 outer membrane protein complex presents a protective humoral immune response; however, its efficacy is variable. Immunodominant epitopes seem to be a key-limiting factor for the adaptive immunity. We have successfully demonstrated that critical motifs of the MSP1a functional epitope are essential for antibody recognition of infected animal sera, but its protective immunity is yet to be tested. We have evaluated two synthetic vaccine formulations against A. marginale, using epitope-based approach in mice. Mice infection with bovine anaplasmosis was demonstrated by qPCR analysis of erythrocytes after 15-day exposure. A proof-of-concept was obtained in this murine model, in which peptides conjugated to bovine serum albumin were used for immunization in three 15-day intervals by intraperitoneal injections before challenging with live bacteria. Blood samples were analyzed for the presence of specific IgG2a and IgG1 antibodies, as well as for the rickettsemia analysis. A panel containing the cytokines' transcriptional profile for innate and adaptive immune responses was carried out through qPCR. Immunized BALB/c mice challenged with A. marginale presented stable body weight, reduced number of infected erythrocytes, and no mortality; and among control groups mortality rates ranged from 15% to 29%. Additionally, vaccines have significantly induced higher IgG2a than IgG1 response, followed by increased expression of pro-inflammatory cytokines. This is a successful demonstration of epitope-based vaccines, and protection against anaplasmosis may be associated with elicitation of effector functions of humoral and cellular immune responses in murine model.
Conflict of interest statement
Figures


Similar articles
-
Mapping of B-cell epitopes in the N-terminal repeated peptides of Anaplasma marginale major surface protein 1a and characterization of the humoral immune response of cattle immunized with recombinant and whole organism antigens.Vet Immunol Immunopathol. 2004 Apr;98(3-4):137-51. doi: 10.1016/j.vetimm.2003.11.003. Vet Immunol Immunopathol. 2004. PMID: 15010223
-
Evaluation of humoral and cellular immune response of BALB/c mice immunized with a recombinant fragment of MSP1a from Anaplasma marginale using carbon nanotubes as a carrier molecule.Vaccine. 2014 Apr 17;32(19):2160-6. doi: 10.1016/j.vaccine.2014.02.062. Epub 2014 Mar 4. Vaccine. 2014. PMID: 24606864
-
A hybrid protein containing MSP1a repeats and Omp7, Omp8 and Omp9 epitopes protect immunized BALB/c mice against anaplasmosis.Vet Res. 2018 Jan 19;49(1):6. doi: 10.1186/s13567-018-0503-4. Vet Res. 2018. PMID: 29351812 Free PMC article.
-
Antigens and alternatives for control of Anaplasma marginale infection in cattle.Clin Microbiol Rev. 2003 Oct;16(4):698-712. doi: 10.1128/CMR.16.4.698-712.2003. Clin Microbiol Rev. 2003. PMID: 14557295 Free PMC article. Review.
-
Strategies to interrupt the development of Anaplasma marginale in its tick vector. The effect of bovine-derived antibodies.Ann N Y Acad Sci. 1996 Jul 23;791:157-65. doi: 10.1111/j.1749-6632.1996.tb53522.x. Ann N Y Acad Sci. 1996. PMID: 8784497 Review.
Cited by
-
Improved serological detection of rheumatoid arthritis: a highly antigenic mimotope of carbonic anhydrase III selected in a murine model by phage display.Arthritis Res Ther. 2015 Jun 23;17(1):168. doi: 10.1186/s13075-015-0685-3. Arthritis Res Ther. 2015. PMID: 26099944 Free PMC article.
-
Construction and immunogenicity of a recombinant swinepox virus expressing a multi-epitope peptide for porcine reproductive and respiratory syndrome virus.Sci Rep. 2017 Mar 8;7:43990. doi: 10.1038/srep43990. Sci Rep. 2017. PMID: 28272485 Free PMC article.
-
Development of a subcutaneous ear implant to deliver an anaplasmosis vaccine to dairy steers.J Anim Sci. 2020 Jun 1;98(6):skz392. doi: 10.1093/jas/skz392. J Anim Sci. 2020. PMID: 31889177 Free PMC article.
-
Anaplasma marginale: Diversity, Virulence, and Vaccine Landscape through a Genomics Approach.Biomed Res Int. 2016;2016:9032085. doi: 10.1155/2016/9032085. Epub 2016 Aug 17. Biomed Res Int. 2016. PMID: 27610385 Free PMC article. Review.
-
Structural and functional characterization of a novel scFv anti-HSP60 of Strongyloides sp.Sci Rep. 2015 May 21;5:10447. doi: 10.1038/srep10447. Sci Rep. 2015. PMID: 25994608 Free PMC article.
References
-
- Kocan KM, de la Fuente J, Blouin EF, Coetzee JF, Ewing SA (2010) The natural history of Anaplasma marginale . Vet Parasitol 167: 95–107. - PubMed
-
- de Andrade GM, Machado RZ, Vidotto MC, Vidotto O (2004) Immunization of bovines using a DNA vaccine (pcDNA3.1/MSP1b) prepared from the Jaboticabal strain of Anaplasma marginale . Ann N Y Acad Sci 1026: 257–266. - PubMed
-
- Kawasaki PM, Kano FS, Tamekuni K, Garcia JL, Marana ER, et al. (2007) Immune response of BALB/c mouse immunized with recombinant MSPs proteins of Anaplasma marginale binding to immunostimulant complex (ISCOM). Res Vet Sci 83: 347–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

