Norepinephrine transporter variant A457P knock-in mice display key features of human postural orthostatic tachycardia syndrome
- PMID: 23580201
- PMCID: PMC3701219
- DOI: 10.1242/dmm.012203
Norepinephrine transporter variant A457P knock-in mice display key features of human postural orthostatic tachycardia syndrome
Abstract
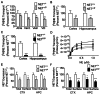
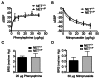
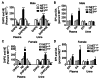
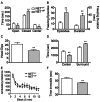
Postural orthostatic tachycardia syndrome (POTS) is a common autonomic disorder of largely unknown etiology that presents with sustained tachycardia on standing, syncope and elevated norepinephrine spillover. Some individuals with POTS experience anxiety, depression and cognitive dysfunction. Previously, we identified a mutation, A457P, in the norepinephrine (NE; also known as noradrenaline) transporter (NET; encoded by SLC6A2) in POTS patients. NET is expressed at presynaptic sites in NE neurons and plays a crucial role in regulating NE signaling and homeostasis through NE reuptake into noradrenergic nerve terminals. Our in vitro studies demonstrate that A457P reduces both NET surface trafficking and NE transport and exerts a dominant-negative impact on wild-type NET proteins. Here we report the generation and characterization of NET A457P mice, demonstrating the ability of A457P to drive the POTS phenotype and behaviors that are consistent with reported comorbidities. Mice carrying one A457P allele (NET(+/P)) exhibited reduced brain and sympathetic NE transport levels compared with wild-type (NET(+/+)) mice, whereas transport activity in mice carrying two A457P alleles (NET(P/P)) was nearly abolished. NET(+/P) and NET(P/P) mice exhibited elevations in plasma and urine NE levels, reduced 3,4-dihydroxyphenylglycol (DHPG), and reduced DHPG:NE ratios, consistent with a decrease in sympathetic nerve terminal NE reuptake. Radiotelemetry in unanesthetized mice revealed tachycardia in NET(+/P) mice without a change in blood pressure or baroreceptor sensitivity, consistent with studies of human NET A457P carriers. NET(+/P) mice also demonstrated behavioral changes consistent with CNS NET dysfunction. Our findings support that NET dysfunction is sufficient to produce a POTS phenotype and introduces the first genetic model suitable for more detailed mechanistic studies of the disorder and its comorbidities.
Figures


References
-
- Alvarez P. A., Pahissa J. (2010). QT alterations in psychopharmacology: proven candidates and suspects. Curr. Drug Saf. 5, 97–104 - PubMed
-
- Barton D. A., Dawood T., Lambert E. A., Esler M. D., Haikerwal D., Brenchley C., Socratous F., Kaye D. M., Schlaich M. P., Hickie I., et al. (2007). Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J. Hypertens. 25, 2117–2124 - PubMed
-
- Beane M., Marrocco R. T. (2004). Norepinephrine and acetylcholine mediation of the components of reflexive attention: implications for attention deficit disorders. Prog. Neurobiol. 74, 167–181 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

