Active transcriptomic and proteomic reprogramming in the C. elegans nucleotide excision repair mutant xpa-1
- PMID: 23580547
- PMCID: PMC3664812
- DOI: 10.1093/nar/gkt225
Active transcriptomic and proteomic reprogramming in the C. elegans nucleotide excision repair mutant xpa-1
Abstract
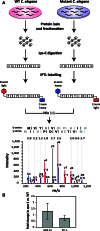
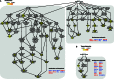
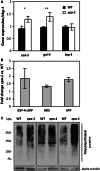
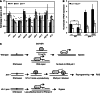
Transcription-blocking oxidative DNA damage is believed to contribute to aging and to underlie activation of oxidative stress responses and down-regulation of insulin-like signaling (ILS) in Nucleotide Excision Repair (NER) deficient mice. Here, we present the first quantitative proteomic description of the Caenorhabditis elegans NER-defective xpa-1 mutant and compare the proteome and transcriptome signatures. Both methods indicated activation of oxidative stress responses, which was substantiated biochemically by a bioenergetic shift involving increased steady-state reactive oxygen species (ROS) and Adenosine triphosphate (ATP) levels. We identify the lesion-detection enzymes of Base Excision Repair (NTH-1) and global genome NER (XPC-1 and DDB-1) as upstream requirements for transcriptomic reprogramming as RNA-interference mediated depletion of these enzymes prevented up-regulation of genes over-expressed in the xpa-1 mutant. The transcription factors SKN-1 and SLR-2, but not DAF-16, were identified as effectors of reprogramming. As shown in human XPA cells, the levels of transcription-blocking 8,5'-cyclo-2'-deoxyadenosine lesions were reduced in the xpa-1 mutant compared to the wild type. Hence, accumulation of cyclopurines is unlikely to be sufficient for reprogramming. Instead, our data support a model where the lesion-detection enzymes NTH-1, XPC-1 and DDB-1 play active roles to generate a genomic stress signal sufficiently strong to result in transcriptomic reprogramming in the xpa-1 mutant.
Figures

References
-
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. - PubMed
-
- Kamileri I, Karakasilioti I, Garinis GA. Nucleotide excision repair: new tricks with old bricks. Trends Genet. 2012;28:566–573. - PubMed
-
- Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

