Menin epigenetically represses Hedgehog signaling in MEN1 tumor syndrome
- PMID: 23580576
- PMCID: PMC4100916
- DOI: 10.1158/0008-5472.CAN-12-3158
Menin epigenetically represses Hedgehog signaling in MEN1 tumor syndrome
Abstract
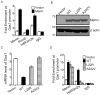
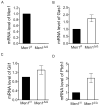
Multiple endocrine neoplasia type 1 (MEN1) is an inherited tumor syndrome that includes susceptibility to pancreatic islet tumors. This syndrome results from mutations in the MEN1 gene, encoding menin. Although menin acts as an oncogenic cofactor for mixed lineage leukemia (MLL) fusion protein-mediated histone H3 lysine 4 methylation, the precise basis for how menin suppresses gene expression and proliferation of pancreatic beta cells remains poorly understood. Here, we show that menin ablation enhances Hedgehog signaling, a proproliferative and oncogenic pathway, in murine pancreatic islets. Menin directly interacts with protein arginine methyltransferase 5 (PRMT5), a negative regulator of gene transcription. Menin recruits PRMT5 to the promoter of the Gas1 gene, a crucial factor for binding of Sonic Hedgehog (Shh) ligand to its receptor PTCH1 and subsequent activation of the Hedgehog signaling pathway, increases repressive histone arginine symmetric dimethylation (H4R3m2s), and suppresses Gas1 expression. Notably, MEN1 disease-related menin mutants have reduced binding to PRMT5, and fail to impart the repressive H4R3m2s mark at the Gas1 promoter, resulting in its elevated expression. Pharmacologic inhibition of Hedgehog signaling significantly reduces proliferation of insulinoma cells, and expression of Hedgehog signaling targets including Ptch1, in MEN1 tumors of mice. These findings uncover a novel link between menin and Hedgehog signaling whereby menin/PRMT5 epigenetically suppresses Hedgehog signaling, revealing it as a target for treating MEN1 tumors.
©2013 AACR.
Conflict of interest statement
Figures
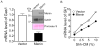



References
-
- Libe R, Bertherat J. Molecular genetics of adrenocortical tumours, from familial to sporadic diseases. Eur J Endocrinol. 2005;153:477–87. - PubMed
-
- Burgess JR, Nord B, David R, et al. Phenotype and phenocopy: the relationship between genotype and clinical phenotype in a single large family with multiple endocrine neoplasia type 1 (MEN 1) Clin Endocrinol (Oxf) 2000;53:205–11. - PubMed
-
- Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer. 2005;5:367–75. - PubMed
-
- Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

