Prolonged inhibition of 5-HT₃ receptors by palonosetron results from surface receptor inhibition rather than inducing receptor internalization
- PMID: 23581504
- PMCID: PMC3831706
- DOI: 10.1111/bph.12204
Prolonged inhibition of 5-HT₃ receptors by palonosetron results from surface receptor inhibition rather than inducing receptor internalization
Abstract
Background and purpose: The 5-HT₃ receptor antagonist palonosetron is an important treatment for emesis and nausea during cancer therapy. Its clinical efficacy may result from its unique binding and clearance characteristics and receptor down-regulation mechanisms. We investigated the mechanisms by which palonosetron exerts its long-term inhibition of 5-HT₃ receptors for a better understanding of its clinical efficacy.
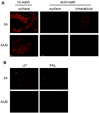
Experimental approach: Cell surface receptors (recombinantly expressed 5HT₃A or 5HT₃AB in COS-7 cells) were monitored using [³H]granisetron binding and ELISA after exposure to palonosetron. Receptor endocytosis was investigated using immunofluorescence microscopy.
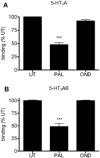
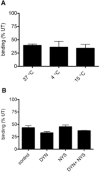
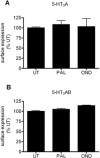
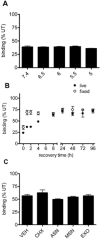
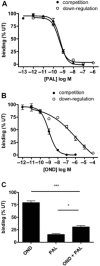
Key results: Chronic exposure to palonosetron reduced the number of available cell surface [³H]granisetron binding sites. This down-regulation was not sensitive to either low temperature or pharmacological inhibitors of endocytosis (dynasore or nystatin) suggesting that internalization did not play a role. This was corroborated by our observation that there was no change in cell surface 5-HT₃ receptor levels or increase in endocytic rate. Palonosetron exhibited slow dissociation from the receptor over many hours, with a significant proportion of binding sites being occupied for at least 4 days. Furthermore, our observations suggest that chronic receptor down-regulation involved interactions with an allosteric binding site.
Conclusions and implications: Palonosetron acts as a pseudo-irreversible antagonist causing prolonged inhibition of 5-HT₃ receptors due to its very slow dissociation. In addition, an irreversible binding mode persists for at least 4 days. Allosteric receptor interactions appear to play a role in this phenomenon.
© 2013 The British Pharmacological Society.
Figures
Similar articles
-
Agonists and antagonists induce different palonosetron dissociation rates in 5-HT₃A and 5-HT₃AB receptors.Neuropharmacology. 2013 Oct;73:241-6. doi: 10.1016/j.neuropharm.2013.05.010. Epub 2013 Jun 5. Neuropharmacology. 2013. PMID: 23747573 Free PMC article.
-
Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function.Eur J Pharmacol. 2010 Jan 25;626(2-3):193-9. doi: 10.1016/j.ejphar.2009.10.002. Epub 2009 Oct 15. Eur J Pharmacol. 2010. PMID: 19836386
-
Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor.Anesth Analg. 2008 Aug;107(2):469-78. doi: 10.1213/ane.0b013e318172fa74. Anesth Analg. 2008. PMID: 18633025
-
Palonosetron: an evidence-based choice in prevention of nausea and vomiting induced by moderately emetogenic chemotherapy.Tumori. 2012 May-Jun;98(3):279-86. doi: 10.1177/030089161209800301. Tumori. 2012. PMID: 22825501 Review.
-
Palonosetron: a second generation 5-hydroxytryptamine 3 receptor antagonist.Expert Opin Drug Metab Toxicol. 2009 Dec;5(12):1577-86. doi: 10.1517/17425250903407289. Expert Opin Drug Metab Toxicol. 2009. PMID: 19929251 Review.
Cited by
-
Agonists and antagonists induce different palonosetron dissociation rates in 5-HT₃A and 5-HT₃AB receptors.Neuropharmacology. 2013 Oct;73:241-6. doi: 10.1016/j.neuropharm.2013.05.010. Epub 2013 Jun 5. Neuropharmacology. 2013. PMID: 23747573 Free PMC article.
-
Structure, Function and Physiology of 5-Hydroxytryptamine Receptors Subtype 3.Subcell Biochem. 2021;96:373-408. doi: 10.1007/978-3-030-58971-4_11. Subcell Biochem. 2021. PMID: 33252737 Review.
-
Higher dose of palonosetron versus lower dose of palonosetron plus droperidol to prevent postoperative nausea and vomiting after eye enucleation and orbital hydroxyapatite implant surgery: a randomized, double-blind trial.Drug Des Devel Ther. 2017 May 15;11:1465-1472. doi: 10.2147/DDDT.S129022. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28553076 Free PMC article. Clinical Trial.
-
Comparison between the antiemetic effects of palonosetron and granisetron in breast cancer patients treated with anthracycline-based regimens.Oncol Lett. 2015 Jan;9(1):119-124. doi: 10.3892/ol.2014.2640. Epub 2014 Oct 24. Oncol Lett. 2015. PMID: 25435944 Free PMC article.
-
Prevention and Treatment of Postoperative Nausea and Vomiting (PONV): A Review of Current Recommendations and Emerging Therapies.Ther Clin Risk Manag. 2020 Dec 31;16:1305-1317. doi: 10.2147/TCRM.S256234. eCollection 2020. Ther Clin Risk Manag. 2020. PMID: 33408475 Free PMC article. Review.
References
-
- Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17:1441–1449. - PubMed
-
- Bollan KA, Baur R, Hales TG, Sigel E, Connolly CN. The promiscuous role of the epsilon subunit in GABAA receptor biogenesis. Mol Cell Neurosci. 2008;37:610–621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

