Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal
- PMID: 23582326
- PMCID: PMC3898614
- DOI: 10.1016/j.cell.2013.03.010
Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal
Abstract
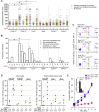
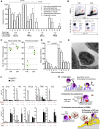
The functions of Nr4a1-dependent Ly6C(low) monocytes remain enigmatic. We show that they are enriched within capillaries and scavenge microparticles from their lumenal side in a steady state. In the kidney cortex, perturbation of homeostasis by a TLR7-dependent nucleic acid "danger" signal, which may signify viral infection or local cell death, triggers Gαi-dependent intravascular retention of Ly6C(low) monocytes by the endothelium. Then, monocytes recruit neutrophils in a TLR7-dependent manner to mediate focal necrosis of endothelial cells, whereas the monocytes remove cellular debris. Prevention of Ly6C(low) monocyte development, crawling, or retention in Nr4a1(-/-), Itgal(-/-), and Tlr7(host-/-BM+/+) and Cx3cr1(-/-) mice, respectively, abolished neutrophil recruitment and endothelial killing. Prevention of neutrophil recruitment in Tlr7(host+/+BM-/-) mice or by neutrophil depletion also abolished endothelial cell necrosis. Therefore, Ly6C(low) monocytes are intravascular housekeepers that orchestrate the necrosis by neutrophils of endothelial cells that signal a local threat sensed via TLR7 followed by the in situ phagocytosis of cellular debris.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





References
-
- Amano H., Amano E., Santiago-Raber M.L., Moll T., Martinez-Soria E., Fossati-Jimack L., Iwamoto M., Rozzo S.J., Kotzin B.L., Izui S. Selective expansion of a monocyte subset expressing the CD11c dendritic cell marker in the Yaa model of systemic lupus erythematosus. Arthritis Rheum. 2005;52:2790–2798. - PubMed
-
- Auffray C., Fogg D., Garfa M., Elain G., Join-Lambert O., Kayal S., Sarnacki S., Cumano A., Lauvau G., Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. - PubMed
-
- Auffray C., Sieweke M.H., Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009;27:669–692. - PubMed
Supplemental References
-
- Boscacci R.T., Pfeiffer F., Gollmer K., Sevilla A.I., Martin A.M., Soriano S.F., Natale D., Henrickson S., von Andrian U.H., Fukui Y. Comprehensive analysis of lymph node stroma-expressed Ig superfamily members reveals redundant and nonredundant roles for ICAM-1, ICAM-2, and VCAM-1 in lymphocyte homing. Blood. 2010;116:915–925. - PMC - PubMed
-
- Ding Z.M., Babensee J.E., Simon S.I., Lu H., Perrard J.L., Bullard D.C., Dai X.Y., Bromley S.K., Dustin M.L., Entman M.L. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 1999;163:5029–5038. - PubMed
-
- Hemmi H., Kaisho T., Takeuchi O., Sato S., Sanjo H., Hoshino K., Horiuchi T., Tomizawa H., Takeda K., Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002;3:196–200. - PubMed
-
- Lee S.L., Wesselschmidt R.L., Linette G.P., Kanagawa O., Russell J.H., Milbrandt J. Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77) Science. 1995;269:532–535. - PubMed
-
- Schaefer B.C., Schaefer M.L., Kappler J.W., Marrack P., Kedl R.M. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell. Immunol. 2001;214:110–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

