Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration
- PMID: 23582327
- PMCID: PMC3663598
- DOI: 10.1016/j.cell.2013.02.053
Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration
Abstract
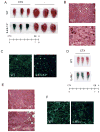
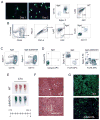
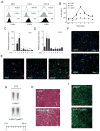
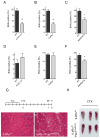
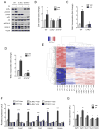
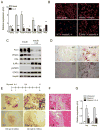
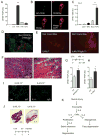
In vertebrates, activation of innate immunity is an early response to injury, implicating it in the regenerative process. However, the mechanisms by which innate signals might regulate stem cell functionality are unknown. Here, we demonstrate that type 2 innate immunity is required for regeneration of skeletal muscle after injury. Muscle damage results in rapid recruitment of eosinophils, which secrete IL-4 to activate the regenerative actions of muscle resident fibro/adipocyte progenitors (FAPs). In FAPs, IL-4/IL-13 signaling serves as a key switch to control their fate and functions. Activation of IL-4/IL-13 signaling promotes proliferation of FAPs to support myogenesis while inhibiting their differentiation into adipocytes. Surprisingly, type 2 cytokine signaling is also required in FAPs, but not in myeloid cells, for rapid clearance of necrotic debris, a process that is necessary for timely and complete regeneration of tissues.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Type 2 immunity: regenerating muscles the type 2 way.Nat Rev Immunol. 2013 Jun;13(6):395. doi: 10.1038/nri3460. Epub 2013 May 7. Nat Rev Immunol. 2013. PMID: 23648971 No abstract available.
References
-
- Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10:844–854. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- K08 HL098361/HL/NHLBI NIH HHS/United States
- DP1AR064158/AR/NIAMS NIH HHS/United States
- R01 DK081405/DK/NIDDK NIH HHS/United States
- DP1 OD006415/OD/NIH HHS/United States
- R37 AG023806/AG/NIA NIH HHS/United States
- T32 HL007731/HL/NHLBI NIH HHS/United States
- DK081405/DK/NIDDK NIH HHS/United States
- R01 AG023806/AG/NIA NIH HHS/United States
- 5T32HL007731/HL/NHLBI NIH HHS/United States
- P01 AG036695/AG/NIA NIH HHS/United States
- DP1 OD000392/OD/NIH HHS/United States
- DP1 AR064158/AR/NIAMS NIH HHS/United States
- R01 AR062185/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

