Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35
- PMID: 23582353
- PMCID: PMC3728272
- DOI: 10.1016/j.ophtha.2013.01.021
Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35
Erratum in
-
Corrigendum.Ophthalmology. 2016 Dec;123(12):2634. doi: 10.1016/j.ophtha.2016.10.026. Ophthalmology. 2016. PMID: 27871398 No abstract available.
Abstract
Objective: To describe the long-term effects (10 years) of the Age-Related Eye Disease Study (AREDS) formulation of high-dose antioxidants and zinc supplement on progression of age-related macular degeneration (AMD).
Design: Multicenter, randomized, controlled, clinical trial followed by an epidemiologic follow-up study.
Participants: We enrolled 4757 participants with varying severity of AMD in the clinical trial; 3549 surviving participants consented to the follow-up study.
Methods: Participants were randomly assigned to antioxidants C, E, and β-carotene and/or zinc versus placebo during the clinical trial. For participants with intermediate or advanced AMD in 1 eye, the AREDS formulation delayed the progression to advanced AMD. Participants were then enrolled in a follow-up study. Eye examinations were conducted with annual fundus photographs and best-corrected visual acuity assessments. Medical histories and mortality were obtained for safety monitoring. Repeated measures logistic regression was used in the primary analyses.
Main outcome measures: Photographic assessment of progression to, or history of treatment for, advanced AMD (neovascular [NV] or central geographic atrophy [CGA]), and moderate visual acuity loss from baseline (≥15 letters).
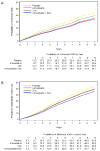
Results: Comparison of the participants originally assigned to placebo in AREDS categories 3 and 4 at baseline with those originally assigned to AREDS formulation at 10 years demonstrated a significant (P<0.001) odds reduction in the risk of developing advanced AMD or the development of NV AMD (odds ratio [OR], 0.66, 95% confidence interval [CI], 0.53-0.83 and OR, 0.60; 95% CI, 0.47-0. 78, respectively). No significant reduction (P = 0.93) was seen for the CGA (OR, 1.02; 95% CI, 0.71-1.45). A significant reduction (P = 0.002) for the development of moderate vision loss was seen (OR 0.71; 95% CI, 0.57-0.88). No adverse effects were associated with the AREDS formulation. Mortality was reduced in participants assigned to zinc, especially death from circulatory diseases.
Conclusions: Five years after the clinical trial ended, the beneficial effects of the AREDS formulation persisted for development of NV AMD but not for CGA. These results are consistent with the original recommendations that persons with intermediate or advanced AMD in 1 eye should consider taking the AREDS formulation.
Financial disclosure(s): The authors have no proprietary or commercial interest in any of the materials discussed in this article.
Copyright © 2013 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
No authors have any financial/conflicting interests to disclose.
Figures
References
-
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. - PubMed
-
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes: 10 years after the Diabetes Control and Complications Trial. Arch Ophthalmol. 2008;126:1707–15. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

