Transglutaminase and polyamination of tubulin: posttranslational modification for stabilizing axonal microtubules
- PMID: 23583110
- PMCID: PMC3627183
- DOI: 10.1016/j.neuron.2013.01.036
Transglutaminase and polyamination of tubulin: posttranslational modification for stabilizing axonal microtubules
Abstract
Neuronal microtubules support intracellular transport, facilitate axon growth, and form a basis for neuronal morphology. While microtubules in nonneuronal cells are depolymerized by cold, Ca(2+), or antimitotic drugs, neuronal microtubules are unusually stable. Such stability is important for normal axon growth and maintenance, while hyperstability may compromise neuronal function in aging and degeneration. Though mechanisms for stability are unclear, studies suggest that stable microtubules contain biochemically distinct tubulins that are more basic than conventional tubulins. Transglutaminase-catalyzed posttranslational incorporation of polyamines is one of the few modifications of intracellular proteins that add positive charges. Here we show that neuronal tubulin can be polyaminated by transglutaminase. Endogenous brain transglutaminase-catalyzed polyaminated tubulins have the biochemical characteristics of neuronal stable microtubules. Inhibiting polyamine synthesis or transglutaminase activity significantly decreases microtubule stability in vitro and in vivo. Together, these findings suggest that transglutaminase-catalyzed polyamination of tubulins stabilizes microtubules essential for unique neuronal structures and functions.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




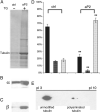

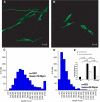
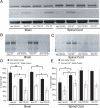
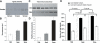
Comment in
-
Microtubule stability in the axon: new answers to an old mystery.Neuron. 2013 Apr 10;78(1):3-5. doi: 10.1016/j.neuron.2013.03.012. Neuron. 2013. PMID: 23583103
-
Stabilization of neuronal connections and the axonal cytoskeleton.Bioarchitecture. 2014 Jan-Feb;4(1):22-4. doi: 10.4161/bioa.28080. Epub 2014 Feb 3. Bioarchitecture. 2014. PMID: 24492417 Free PMC article.
References
-
- Bailey CD, Johnson GV. Developmental regulation of tissue transglutaminase in the mouse forebrain. J Neurochem. 2004;91:1369–1379. - PubMed
-
- Bailey CD, Tucholski J, Johnson GV. Transglutaminases in neurodegenerative disorders. Prog Exp Tumor Res. 2005;38:139–157. - PubMed
-
- Brady ST. Axonal Dynamics and Regeneration. In: Gorio A, editor. Neuroregeneration. Raven Press; New York City, NY: 1993. pp. 7–36.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

