Developmental regulation and activity-dependent maintenance of GABAergic presynaptic inhibition onto rod bipolar cell axonal terminals
- PMID: 23583111
- PMCID: PMC3627184
- DOI: 10.1016/j.neuron.2013.01.037
Developmental regulation and activity-dependent maintenance of GABAergic presynaptic inhibition onto rod bipolar cell axonal terminals
Abstract
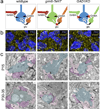
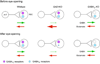
Presynaptic inhibition onto axons regulates neuronal output, but how such inhibitory synapses develop and are maintained in vivo remains unclear. Axon terminals of glutamatergic retinal rod bipolar cells (RBCs) receive GABAA and GABAC receptor-mediated synaptic inhibition. We found that perturbing GABAergic or glutamatergic neurotransmission does not prevent GABAergic synaptogenesis onto RBC axons. But, GABA release is necessary for maintaining axonal GABA receptors. This activity-dependent process is receptor subtype specific: GABAC receptors are maintained, whereas GABAA receptors containing α1, but not α3, subunits decrease over time in mice with deficient GABA synthesis. GABAA receptor distribution on RBC axons is unaffected in GABAC receptor knockout mice. Thus, GABAA and GABAC receptor maintenance are regulated separately. Although immature RBCs elevate their glutamate release when GABA synthesis is impaired, homeostatic mechanisms ensure that the RBC output operates within its normal range after eye opening, perhaps to regain proper visual processing within the scotopic pathway.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Development of presynaptic inhibition onto retinal bipolar cell axon terminals is subclass-specific.J Neurophysiol. 2008 Jul;100(1):304-16. doi: 10.1152/jn.90202.2008. Epub 2008 Apr 24. J Neurophysiol. 2008. PMID: 18436633 Free PMC article.
-
LRRTM4: A Novel Regulator of Presynaptic Inhibition and Ribbon Synapse Arrangements of Retinal Bipolar Cells.Neuron. 2020 Mar 18;105(6):1007-1017.e5. doi: 10.1016/j.neuron.2019.12.028. Epub 2020 Jan 20. Neuron. 2020. PMID: 31974009 Free PMC article.
-
Early synapse formation in developing interneurons of the adult olfactory bulb.J Neurosci. 2009 Dec 2;29(48):15039-52. doi: 10.1523/JNEUROSCI.3034-09.2009. J Neurosci. 2009. PMID: 19955355 Free PMC article.
-
GABAC receptor-mediated inhibition in the retina.Vision Res. 2004 Dec;44(28):3289-96. doi: 10.1016/j.visres.2004.07.023. Vision Res. 2004. PMID: 15535996 Review.
-
Synaptic release at mammalian bipolar cell terminals.Vis Neurosci. 2011 Jan;28(1):109-19. doi: 10.1017/S0952523810000453. Vis Neurosci. 2011. PMID: 21272392 Free PMC article. Review.
Cited by
-
Eliminating Glutamatergic Input onto Horizontal Cells Changes the Dynamic Range and Receptive Field Organization of Mouse Retinal Ganglion Cells.J Neurosci. 2018 Feb 21;38(8):2015-2028. doi: 10.1523/JNEUROSCI.0141-17.2018. Epub 2018 Jan 19. J Neurosci. 2018. PMID: 29352045 Free PMC article.
-
Developmental expression of α- and β- synuclein in the mouse retina.Brain Struct Funct. 2025 Jul 17;230(7):120. doi: 10.1007/s00429-025-02984-8. Brain Struct Funct. 2025. PMID: 40673949
-
Sensory deprivation arrests cellular and synaptic development of the night-vision circuitry in the retina.Curr Biol. 2023 Oct 23;33(20):4415-4429.e3. doi: 10.1016/j.cub.2023.08.087. Epub 2023 Sep 27. Curr Biol. 2023. PMID: 37769662 Free PMC article.
-
Muscle afferent excitability testing in spinal root-intact rats: dissociating peripheral afferent and efferent volleys generated by intraspinal microstimulation.J Neurophysiol. 2017 Feb 1;117(2):796-807. doi: 10.1152/jn.00874.2016. Epub 2016 Dec 14. J Neurophysiol. 2017. PMID: 27974451 Free PMC article.
-
Activity-dependent adaptations in inhibitory axons.Front Cell Neurosci. 2013 Nov 21;7:219. doi: 10.3389/fncel.2013.00219. Front Cell Neurosci. 2013. PMID: 24312009 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

