Torsin mediates primary envelopment of large ribonucleoprotein granules at the nuclear envelope
- PMID: 23583177
- PMCID: PMC3683601
- DOI: 10.1016/j.celrep.2013.03.015
Torsin mediates primary envelopment of large ribonucleoprotein granules at the nuclear envelope
Abstract
A previously unrecognized mechanism through which large ribonucleoprotein (megaRNP) granules exit the nucleus is by budding through the nuclear envelope (NE). This mechanism is akin to the nuclear egress of herpes-type viruses and is essential for proper synapse development. However, the molecular machinery required to remodel the NE during this process is unknown. Here, we identify Torsin, an AAA-ATPase that in humans is linked to dystonia, as a major mediator of primary megaRNP envelopment during NE budding. In torsin mutants, megaRNPs accumulate within the perinuclear space, and the messenger RNAs contained within fail to reach synaptic sites, preventing normal synaptic protein synthesis and thus proper synaptic bouton development. These studies begin to establish the cellular machinery underlying the exit of megaRNPs via budding, offer an explanation for the "nuclear blebbing" phenotype found in dystonia models, and provide an important link between Torsin and the synaptic phenotypes observed in dystonia.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
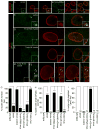
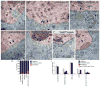
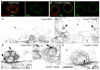
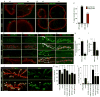
References
-
- Barco A, Lopez de Armentia M, Alarcon JM. Synapse-specific stabilization of plasticity processes: the synaptic tagging and capture hypothesis revisited 10 years later. Neurosci Biobehav Rev. 2008;32:831–851. - PubMed
-
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

