A rapid fluorescent method to quantify neuronal loss after experimental intracerebral hemorrhage
- PMID: 23583700
- PMCID: PMC3679307
- DOI: 10.1016/j.jneumeth.2013.03.025
A rapid fluorescent method to quantify neuronal loss after experimental intracerebral hemorrhage
Abstract
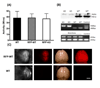
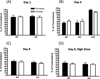
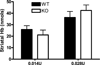
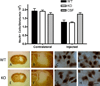
Neuronal loss in tissue surrounding an intracerebral hemorrhage (ICH) is usually quantified by labor-intensive histological methods that are subject to bias. Fluorescent protein expression has been successfully used as a marker of cell viability in vitro and in retinal studies in vivo, but not in any ICH model to date. The potential of this approach was investigated using transgenic mice that constitutively express the red fluorescent protein variant dTomato in central neurons under the control of the Thy1 promoter. Breeding and growth of these mice were similar to their wild-type counterparts; behavioral phenotyping by digital analysis of home cage video recordings detected no differences. Bright fluorescence was evident in fresh brain samples with minimal background fluorescence, and was reduced in tissue surrounding the hematoma. In order to assess fluorescence loss as an injury marker in a planned study, these mice were crossed with heme oxygenase (HO)-2 knockouts and wild-type controls; striatal hemorrhage was induced by stereotactic injection of collagenase. Fluorescence in hemorrhagic striata was reduced to 86.4±3.9%, 62.2±5.1%, and 58.3±3.0% of contra-lateral on days 1, 4 and 8, respectively, and correlated closely with reduction in striatal cell viability as quantified by MTT assay. HO-2 knockout and wild-type values did not differ significantly. Similar results were observed with stereological cell counts of striatal neurons identified by NeuN immunoreactivity. These results suggest that loss of constitutive dTomato fluorescence is an accurate and efficient marker of neuronal loss in tissue surrounding a striatal hematoma.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures




References
-
- Anegawa NJ, Guttmann RP, Grant ER, Anand R, Lindstrom J, Lynch DR. N-Methyl-D-aspartate receptor mediated toxicity in nonneuronal cell lines: characterization using fluorescent measures of cell viability and reactive oxygen species production. Brain Res Mol Brain Res. 2000;77:163–175. - PubMed
-
- Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H. HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol. 2006;291:C897–C908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

