Thermosensitive liposomes for localized delivery and triggered release of chemotherapy
- PMID: 23583706
- PMCID: PMC5127786
- DOI: 10.1016/j.jconrel.2013.03.036
Thermosensitive liposomes for localized delivery and triggered release of chemotherapy
Abstract
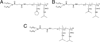
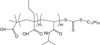
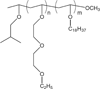
Liposomes are a promising class of nanomedicine with the potential to provide site-specific chemotherapy, thus improving the quality of cancer patient care. First-generation liposomes have emerged as one of the first nanomedicines used clinically for localized delivery of chemotherapy. Second-generation liposomes, i.e. stimuli-responsive liposomes, have the potential to not only provide site-specific chemotherapy, but also triggered drug release and thus greater spatial and temporal control of therapy. Temperature-sensitive liposomes are an especially attractive option, as tumors can be heated in a controlled and predictable manner with external energy sources. Traditional thermosensitive liposomes are composed of lipids that undergo a gel-to-liquid phase transition at several degrees above physiological temperature. More recently, temperature-sensitization of liposomes has been demonstrated with the use of lysolipids and synthetic temperature-sensitive polymers. The design, drug release behavior, and clinical potential of various temperature-sensitive liposomes, as well as the various heating modalities used to trigger release, are discussed in this review.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures









References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Skeel RT, Khleif SN. Handbook of Cancer Chemotherapy. 8th. Philadelphia: Lippincott Williams and Wilkins; 2011.
-
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discovery. 2006;5:219–234. - PubMed
-
- Eliaz RE, Szoka CF., Jr Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res. 2001;61:2592–2601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

