Dietary nimodipine delays the onset of methylmercury neurotoxicity in mice
- PMID: 23583802
- PMCID: PMC3696396
- DOI: 10.1016/j.neuro.2013.03.011
Dietary nimodipine delays the onset of methylmercury neurotoxicity in mice
Abstract
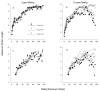
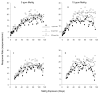
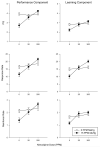
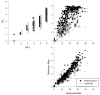
Adult-onset methylmercury (MeHg) exposure is thought to result primarily in sensory and motor deficits but effects on learning are poorly understood. One mechanism by which chronic MeHg may exert its neurotoxicity is via sustained disruption of intracellular calcium homeostasis, with a consequent increase of intracellular Ca(2+) ions in vulnerable neurons. A biochemically heterogeneous group of compounds, calcium channel blockers, have been shown in vitro to attenuate MeHg's toxicity. To evaluate the role of calcium antagonism in MeHg toxicity in vivo, adult BALB/c mice were exposed chronically to 0 or 15 ppm of Hg (as MeHg) via drinking water and to nimodipine, a dihydropryidine, L-type Ca(2+) channel blocker with action in the CNS. Nimodipine was administered orally in diets (0, 20, or 200 ppm, producing approximately 0, 2, or 20 mg/kg/day of nimodipine). An incremental repeated acquisition (IRA) of response chains procedure was used to detect MeHg-induced deficits in learning or motoric function and to evaluate possible neuroprotection by nimodipine. MeHg impaired performance on the IRA task, and this was partially or completely blocked by dietary nimodipine, depending on dose. Measures of learning co-varied with measures of motoric function as indicated by overall response rate. Nimodipine delayed or prevented the behavioral toxicity of MeHg exposure as evidenced by IRA performance; effects on learning seemed secondary to response rate decreases.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Aging, motor function, and sensitivity to calcium channel blockers: An investigation using chronic methylmercury exposure.Behav Brain Res. 2016 Dec 15;315:103-14. doi: 10.1016/j.bbr.2016.07.049. Epub 2016 Jul 30. Behav Brain Res. 2016. PMID: 27481695
-
A microstructural analysis distinguishes motor and motivational influences over voluntary running in animals chronically exposed to methylmercury and nimodipine.Neurotoxicology. 2016 May;54:127-139. doi: 10.1016/j.neuro.2016.04.009. Epub 2016 Apr 16. Neurotoxicology. 2016. PMID: 27095634
-
A bout analysis reveals age-related methylmercury neurotoxicity and nimodipine neuroprotection.Behav Brain Res. 2016 Sep 15;311:147-159. doi: 10.1016/j.bbr.2016.05.032. Epub 2016 May 16. Behav Brain Res. 2016. PMID: 27196441 Free PMC article.
-
Methylmercury and nutrition: adult effects of fetal exposure in experimental models.Neurotoxicology. 2008 Sep;29(5):783-801. doi: 10.1016/j.neuro.2008.06.007. Epub 2008 Jul 5. Neurotoxicology. 2008. PMID: 18652843 Free PMC article. Review.
-
Methylmercury-Induced Neurotoxicity: Focus on Pro-oxidative Events and Related Consequences.Adv Neurobiol. 2017;18:267-286. doi: 10.1007/978-3-319-60189-2_13. Adv Neurobiol. 2017. PMID: 28889272 Review.
Cited by
-
Nimodipine promotes neurite outgrowth and protects against neurotoxicity in PC12 cells.Iran J Basic Med Sci. 2021 Jan;24(1):51-57. doi: 10.22038/ijbms.2020.48567.11152. Iran J Basic Med Sci. 2021. PMID: 33643570 Free PMC article.
-
Neuroprotective and neuroregenerative effects of nimodipine in a model system of neuronal differentiation and neurite outgrowth.Molecules. 2015 Jan 9;20(1):1003-13. doi: 10.3390/molecules20011003. Molecules. 2015. PMID: 25584831 Free PMC article.
-
d-Amphetamine and methylmercury exposure during adolescence alters sensitivity to monoamine uptake inhibitors in adult mice.Neurotoxicology. 2019 May;72:61-73. doi: 10.1016/j.neuro.2019.02.001. Epub 2019 Feb 12. Neurotoxicology. 2019. PMID: 30769003 Free PMC article.
-
Inhibition of voltage-gated calcium channels as common mode of action for (mixtures of) distinct classes of insecticides.Toxicol Sci. 2014 Sep;141(1):103-11. doi: 10.1093/toxsci/kfu110. Epub 2014 Jun 9. Toxicol Sci. 2014. PMID: 24913802 Free PMC article.
-
The catecholaminergic neurotransmitter system in methylmercury-induced neurotoxicity.Adv Neurotoxicol. 2017;1:47-81. doi: 10.1016/bs.ant.2017.07.002. Epub 2017 Sep 1. Adv Neurotoxicol. 2017. PMID: 32346666 Free PMC article. No abstract available.
References
-
- Atchison WD, Hare MF. Mechanisms of methylmercury-induced neurotoxicity. FASEB J. 1994;8:622–9. - PubMed
-
- Atchison WD. Effects of toxic environmental contaminants on voltage-gated calcium channel function: From past to present. J Bioenerg Biomembr. 2003;35:507–32. - PubMed
-
- Bailey JM, Johnson JE, Newland MC. Mechanisms and performance measures in mastery-based incremental repeated acquisition: behavioral and pharmacological analyses. Psychopharmacology. 2010;209:331–41. - PubMed
-
- Bates DM, DebRoy S. Linear mixed models and penalized least squares. J Multivariate Analysis. 2004;91:1–17.
-
- Bearrs JJ, Limke TL, Atchison WD. Methylmercury (MeHg) causes calcium release from smooth endoplasmic reticulum (SER) inositol-1,4,5-triphosphate receptors in rat cerebellar granule neurons. The Toxicologist. 2001;60:184.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

