Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease
- PMID: 23583836
- PMCID: PMC4058883
- DOI: 10.1016/j.yjmcc.2013.04.001
Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease
Erratum in
-
Corrigendum to "Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease" [J. Mol. Cell. Cardiol. 60 (2013) 27-35].J Mol Cell Cardiol. 2018 Aug;121:307. doi: 10.1016/j.yjmcc.2018.04.013. Epub 2018 May 31. J Mol Cell Cardiol. 2018. PMID: 29778253 No abstract available.
Abstract
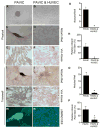
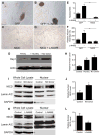
The mature aortic valve is composed of a structured trilaminar extracellular matrix that is interspersed with aortic valve interstitial cells (AVICs) and covered by endothelium. Dysfunction of the valvular endothelium initiates calcification of neighboring AVICs leading to calcific aortic valve disease (CAVD). The molecular mechanism by which endothelial cells communicate with AVICs and cause disease is not well understood. Using a co-culture assay, we show that endothelial cells secrete a signal to inhibit calcification of AVICs. Gain or loss of nitric oxide (NO) prevents or accelerates calcification of AVICs, respectively, suggesting that the endothelial cell-derived signal is NO. Overexpression of Notch1, which is genetically linked to human CAVD, retards the calcification of AVICs that occurs with NO inhibition. In AVICs, NO regulates the expression of Hey1, a downstream target of Notch1, and alters nuclear localization of Notch1 intracellular domain. Finally, Notch1 and NOS3 (endothelial NO synthase) display an in vivo genetic interaction critical for proper valve morphogenesis and the development of aortic valve disease. Our data suggests that endothelial cell-derived NO is a regulator of Notch1 signaling in AVICs in the development of the aortic valve and adult aortic valve disease.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None.
Figures



References
-
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

