A role of TARPs in the expression and plasticity of calcium-permeable AMPARs: evidence from cerebellar neurons and glia
- PMID: 23583927
- PMCID: PMC3751754
- DOI: 10.1016/j.neuropharm.2013.03.037
A role of TARPs in the expression and plasticity of calcium-permeable AMPARs: evidence from cerebellar neurons and glia
Abstract
The inclusion of GluA2 subunits has a profound impact on the channel properties of AMPA receptors (AMPARs), in particular rendering them impermeable to calcium. While GluA2-containing AMPARs are the most abundant in the central nervous system, GluA2-lacking calcium-permeable AMPARs are also expressed in wide variety of neurons and glia. Accumulating evidence suggests that the dynamic control of the GluA2 content of AMPARs plays a critical role in development, synaptic plasticity, and diverse neurological conditions ranging from ischemia-induced brain damage to drug addiction. It is thus important to understand the molecular mechanisms involved in regulating the balance of AMPAR subtypes, particularly the role of their co-assembled auxiliary subunits. The discovery of transmembrane AMPAR regulatory proteins (TARPs), initially within the cerebellum, has transformed the field of AMPAR research. It is now clear that these auxiliary subunits play a key role in multiple aspects of AMPAR trafficking and function in the brain. Yet, their precise role in AMPAR subtype-specific regulation has only recently received particular attention. Here we review recent findings on the differential regulation of calcium-permeable (CP-) and -impermeable (CI-) AMPARs in cerebellar neurons and glial cells, and discuss the critical involvement of TARPs in this process. This article is part of the Special Issue entitled 'Glutamate Receptor-Dependent Synaptic Plasticity'.
Keywords: AMPA receptors; Calcium-permeable AMPA receptors; Cerebellum; Glutamate receptors; Plasticity; Synaptic transmission; TARPs.
Copyright © 2013 Elsevier Ltd. All rights reserved.
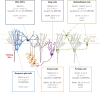
Figures

References
-
- Bedoukian M.A., Weeks A.M., Partin K.M. Different domains of the AMPA receptor direct stargazin-mediated trafficking and stargazin-mediated modulation of kinetics. J. Biol. Chem. 2006;281:23908–23921. - PubMed
-
- Bellone C., Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat. Neurosci. 2006;9:636–641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

