Host and bacterial factors that regulate LC3 recruitment to Listeria monocytogenes during the early stages of macrophage infection
- PMID: 23584039
- PMCID: PMC3722333
- DOI: 10.4161/auto.24406
Host and bacterial factors that regulate LC3 recruitment to Listeria monocytogenes during the early stages of macrophage infection
Abstract
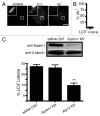
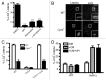
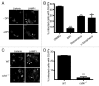
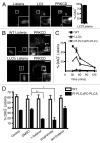
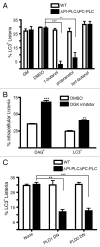
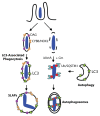
Listeria monocytogenes is a bacterial pathogen that can escape the phagosome and replicate in the cytosol of host cells during infection. We previously observed that a population (up to 35%) of L. monocytogenes strain 10403S colocalize with the macroautophagy marker LC3 at 1 h postinfection. This is thought to give rise to spacious Listeria-containing phagosomes (SLAPs), a membrane-bound compartment harboring slow-growing bacteria that is associated with persistent infection. Here, we examined the host and bacterial factors that mediate LC3 recruitment to bacteria at 1 h postinfection. At this early time point, LC3(+) bacteria were present within single-membrane phagosomes that are LAMP1(+). Protein ubiquitination is known to play a role in targeting cytosolic L. monocytogenes to macroautophagy. However, we found that neither protein ubiquitination nor the ubiquitin-binding adaptor SQSTM1/p62 are associated with LC3(+) bacteria at 1 h postinfection. Reactive oxygen species (ROS) production by the CYBB/NOX2 NADPH oxidase was also required for LC3 recruitment to bacteria at 1 h postinfection and for subsequent SLAP formation. Diacylglycerol is an upstream activator of the CYBB/NOX2 NADPH oxidase, and its production by both bacterial and host phospholipases was required for LC3 recruitment to bacteria. Our data suggest that the LC3-associated phagocytosis (LAP) pathway, which is distinct from macroautophagy, targets L. monocytogenes during the early stage of infection within host macrophages and allows establishment of an intracellular niche (SLAPs) associated with persistent infection.
Keywords: LC3; LC3-associated phagocytosis; Listeria monocytogenes; autophagy; diacylglycerol; innate immunity; reactive oxygen species; ubiquitin.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
