Induction of the metabolic regulator Txnip in fasting-induced and natural torpor
- PMID: 23584857
- PMCID: PMC3740491
- DOI: 10.1210/en.2012-2051
Induction of the metabolic regulator Txnip in fasting-induced and natural torpor
Abstract
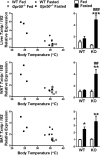
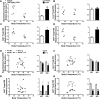
Torpor is a physiological state characterized by controlled lowering of metabolic rate and core body temperature, allowing substantial energy savings during periods of reduced food availability or harsh environmental conditions. The hypothalamus coordinates energy homeostasis and thermoregulation and plays a key role in directing torpor. We recently showed that mice lacking the orphan G protein-coupled receptor Gpr50 readily enter torpor in response to fasting and have now used these mice to conduct a microarray analysis of hypothalamic gene expression changes related to the torpor state. This revealed a strong induction of thioredoxin-interacting protein (Txnip) in the hypothalamus of torpid mice, which was confirmed by quantitative RT-PCR and Western blot analyses. In situ hybridization identified the ependyma lining the third ventricle as the principal site of torpor-related expression of Txnip. To characterize further the relationship between Txnip and torpor, we profiled Txnip expression in mice during prolonged fasting, cold exposure, and 2-deoxyglucose-induced hypometabolism, as well as in naturally occurring torpor bouts in the Siberian hamster. Strikingly, pronounced up-regulation of Txnip expression was only observed in wild-type mice when driven into torpor and during torpor in the Siberian hamster. Increase of Txnip was not limited to the hypothalamus, with exaggerated expression in white adipose tissue, brown adipose tissue, and liver also demonstrated in torpid mice. Given the recent identification of Txnip as a molecular nutrient sensor important in the regulation of energy metabolism, our data suggest that elevated Txnip expression is critical to regulating energy expenditure and fuel use during the extreme hypometabolic state of torpor.
Figures



Comment in
-
Txnip, tanycytes, and torpor.Endocrinology. 2013 Jun;154(6):1970-2. doi: 10.1210/en.2013-1390. Endocrinology. 2013. PMID: 23687114 No abstract available.
Similar articles
-
A role for the melatonin-related receptor GPR50 in leptin signaling, adaptive thermogenesis, and torpor.Curr Biol. 2012 Jan 10;22(1):70-7. doi: 10.1016/j.cub.2011.11.043. Epub 2011 Dec 22. Curr Biol. 2012. PMID: 22197240
-
Nutrient-sensing hypothalamic TXNIP links nutrient excess to energy imbalance in mice.J Neurosci. 2011 Apr 20;31(16):6019-27. doi: 10.1523/JNEUROSCI.6498-10.2011. J Neurosci. 2011. PMID: 21508227 Free PMC article.
-
TXNIP in Agrp neurons regulates adiposity, energy expenditure, and central leptin sensitivity.J Neurosci. 2012 Jul 18;32(29):9870-7. doi: 10.1523/JNEUROSCI.0353-12.2012. J Neurosci. 2012. PMID: 22815502 Free PMC article.
-
The Art of Chilling Out: How Neurons Regulate Torpor.Bioessays. 2025 Feb;47(2):e202400190. doi: 10.1002/bies.202400190. Epub 2024 Nov 26. Bioessays. 2025. PMID: 39600072 Free PMC article. Review.
-
Seasonal Control of Mammalian Energy Balance: Recent Advances in the Understanding of Daily Torpor and Hibernation.J Neuroendocrinol. 2016 Nov;28(11). doi: 10.1111/jne.12437. J Neuroendocrinol. 2016. PMID: 27755687 Review.
Cited by
-
Central activation of the A1 adenosine receptor in fed mice recapitulates only some of the attributes of daily torpor.J Comp Physiol B. 2017 Jul;187(5-6):835-845. doi: 10.1007/s00360-017-1084-7. Epub 2017 Apr 4. J Comp Physiol B. 2017. PMID: 28378088 Free PMC article.
-
Dual regulation of TxNIP by ChREBP and FoxO1 in liver.iScience. 2021 Feb 20;24(3):102218. doi: 10.1016/j.isci.2021.102218. eCollection 2021 Mar 19. iScience. 2021. PMID: 33748706 Free PMC article.
-
Orchestration of gene expression across the seasons: Hypothalamic gene expression in natural photoperiod throughout the year in the Siberian hamster.Sci Rep. 2016 Jul 11;6:29689. doi: 10.1038/srep29689. Sci Rep. 2016. PMID: 27406810 Free PMC article.
-
Antipsychotic inductors of brain hypothermia and torpor-like states: perspectives of application.Psychopharmacology (Berl). 2017 Jan;234(2):173-184. doi: 10.1007/s00213-016-4496-2. Epub 2016 Dec 8. Psychopharmacology (Berl). 2017. PMID: 27933367 Review.
-
Effect of exercise on photoperiod-regulated hypothalamic gene expression and peripheral hormones in the seasonal Dwarf Hamster Phodopus sungorus.PLoS One. 2014 Mar 6;9(3):e90253. doi: 10.1371/journal.pone.0090253. eCollection 2014. PLoS One. 2014. PMID: 24603871 Free PMC article.
References
-
- Bechtold DA, Sidibe A, Saer BR, et al. A role for the melatonin-related receptor GPR50 in leptin signaling, adaptive thermogenesis, and torpor. Curr Biol. 2012;22:70–77 - PubMed
-
- Ivanova EA, Bechtold DA, Dupré SM, et al. Altered metabolism in the melatonin-related receptor (GPR50) knockout mouse. Am J Physiol Endocrinol Metab. 2008;294:E176–E182 - PubMed
-
- Grünewald E, Kinnell HL, Porteous DJ, Thomson PA. GPR50 interacts with neuronal NOGO-A and affects neurite outgrowth. Mol Cell Neurosci. 2009;42:363–371 - PubMed
-
- Thomson PA, Wray NR, Thomson AM, et al. Sex-specific association between bipolar affective disorder in women and GPR50, an X-linked orphan G protein-coupled receptor. Mol Psychiatry. 2005;10:470–478 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

