Nitric oxide regulates vascular adaptive mitochondrial dynamics
- PMID: 23585138
- PMCID: PMC3680775
- DOI: 10.1152/ajpheart.00987.2012
Nitric oxide regulates vascular adaptive mitochondrial dynamics
Abstract
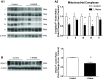
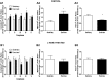
Cardiovascular disease risk factors, such as diabetes, hypertension, dyslipidemia, obesity, and physical inactivity, are all correlated with impaired endothelial nitric oxide synthase (eNOS) function and decreased nitric oxide (NO) production. NO-mediated regulation of mitochondrial biogenesis has been established in many tissues, yet the role of eNOS in vascular mitochondrial biogenesis and dynamics is unclear. We hypothesized that genetic eNOS deletion and 3-day nitric oxide synthase (NOS) inhibition in rodents would result in impaired mitochondrial biogenesis and defunct fission/fusion and autophagy profiles within the aorta. We observed a significant, eNOS expression-dependent decrease in mitochondrial electron transport chain (ETC) protein subunits from complexes I, II, III, and V in eNOS heterozygotes and eNOS null mice compared with age-matched controls. In response to NOS inhibition with NG-nitro-L-arginine methyl ester (L-NAME) treatment in Sprague Dawley rats, significant decreases were observed in ETC protein subunits from complexes I, III, and IV as well as voltage-dependent anion channel 1. Decreased protein content of upstream regulators of mitochondrial biogenesis, cAMP response element-binding protein and peroxisome proliferator-activated receptor-γ coactivator-1α, were observed in response to 3-day L-NAME treatment. Both genetic eNOS deletion and NOS inhibition resulted in decreased manganese superoxide dismutase protein. L-NAME treatment resulted in significant changes to mitochondrial dynamic protein profiles with decreased fusion, increased fission, and minimally perturbed autophagy. In addition, L-NAME treatment blocked mitochondrial adaptation to an exercise intervention in the aorta. These results suggest that eNOS/NO play a role in basal and adaptive mitochondrial biogenesis in the vasculature and regulation of mitochondrial turnover.
Keywords: endothelial nitric oxide synthase; vascular mitochondria.
Figures




References
-
- Abhikit S, Bhaskaran R, Narayanasamy A, Chakroborty A, Manickam N, Dixit M, Mohan V, Blasubramanyam M. Hyperinsulinemia-induced vascular smooth muscle cell migration and proliferation is mediated by converging mechanisms of mitochondrial dysfunction and oxidative stress. Mol Cell Biochem 373: 95–105, 2013 - PubMed
-
- Aizawa K, Shoemaker JK, Overend TJ, Petrella RJ. Effects of lifestyle modification on central artery stiffness in metabolic syndrome subjects with pre-hypertension and/or prediabetes. Diabetes Res Clin Pract 83: 249–256, 2009 - PubMed
-
- Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation 106: 544–549, 2002 - PubMed
-
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

