Symptomatic reduction in free testosterone levels secondary to crizotinib use in male cancer patients
- PMID: 23585220
- PMCID: PMC3686910
- DOI: 10.1002/cncr.28089
Symptomatic reduction in free testosterone levels secondary to crizotinib use in male cancer patients
Abstract
Background: Crizotinib is a tyrosine kinase inhibitor active against ALK, MET, and ROS1. We previously reported that crizotinib decreases testosterone in male patients. The detailed etiology of the effect, its symptomatic significance, and the effectiveness of subsequent testosterone replacement have not been previously reported.
Methods: Male cancer patients treated with crizotinib had total testosterone levels measured and results compared with non-crizotinib-treated patients. Albumin, sex hormone-binding globulin (SHBG), follicle-stimulating hormone (FSH), and/or luteinizing hormone (LH) were tracked longitudinally. A subset of patients had free testosterone levels measured and a hypogonadal screening questionnaire administered. Patients receiving subsequent testosterone supplementation were assessed for symptomatic improvement.
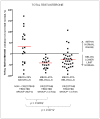
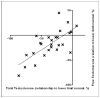
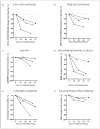
Results: Mean total testosterone levels were -25% below the lower limit of normal (LLN) in 32 crizotinib-treated patients (27 of 32 patients below LLN, 84%) compared with +29% above LLN in 19 non-crizotinib-treated patients (6 of 19 below LLN, 32%), P = .0012. Levels of albumin and SHBG (which both bind testosterone) declined rapidly with crizotinib, but so did FSH, LH, and free testosterone, suggesting a centrally mediated, true hypogonadal effect. Mean free testosterone levels were -17% below LLN (19 of 25 patients below LLN, 76%). Eighty-four percent (16 of 19) with low free levels, and 79% (19/24) with low total levels had symptoms of androgen deficiency. Five of 9 patients (55%) with low testosterone given testosterone supplementation had improvement in symptoms, coincident with increases in testosterone above LLN.
Conclusions: Symptoms of androgen deficiency and free or total/free testosterone levels should be tracked in male patients on crizotinib with consideration of testosterone replacement as appropriate.
Copyright © 2013 American Cancer Society.
Figures

Similar articles
-
Rapid-onset hypogonadism secondary to crizotinib use in men with metastatic nonsmall cell lung cancer.Cancer. 2012 Nov 1;118(21):5302-9. doi: 10.1002/cncr.27450. Epub 2012 Apr 4. Cancer. 2012. PMID: 22488744
-
Multiple Endocrine Disruption by the MET/ALK Inhibitor Crizotinib in Patients With Non-small Cell Lung Cancer.Am J Clin Oncol. 2015 Oct;38(5):442-7. doi: 10.1097/COC.0b013e3182a46896. Am J Clin Oncol. 2015. PMID: 23934135 Free PMC article. Clinical Trial.
-
Hypogonadism related to crizotinib therapy: implications for patient care.Cancer. 2012 Nov 1;118(21):E1-2. doi: 10.1002/cncr.27561. Epub 2012 Apr 4. Cancer. 2012. PMID: 22487894 No abstract available.
-
[ALK-rearranged non-small cell lung cancer: how to optimize treatment with crizotinib in routine practice?].Bull Cancer. 2014 Sep;101(9):823-31. doi: 10.1684/bdc.2014.1976. Bull Cancer. 2014. PMID: 25299566 French.
-
Current and developing therapies for the treatment of non-small cell lung cancer with ALK abnormalities: update and perspectives for clinical practice.Expert Opin Pharmacother. 2016 Dec;17(17):2253-2266. doi: 10.1080/14656566.2016.1242578. Epub 2016 Oct 8. Expert Opin Pharmacother. 2016. PMID: 27682212 Review.
Cited by
-
The Effect of Targeted Therapy for Genitourinary Malignancies on Sexual Function and Fertility.Curr Urol Rep. 2017 Aug;18(8):65. doi: 10.1007/s11934-017-0704-9. Curr Urol Rep. 2017. PMID: 28712040 Review.
-
Treating ALK-positive non-small cell lung cancer.Ann Transl Med. 2018 Apr;6(8):141. doi: 10.21037/atm.2017.11.34. Ann Transl Med. 2018. PMID: 29862230 Free PMC article. Review.
-
When and when not to use testosterone for palliation in cancer care.Curr Oncol Rep. 2014 Apr;16(4):378. doi: 10.1007/s11912-014-0378-0. Curr Oncol Rep. 2014. PMID: 24510740 Review.
-
Managing treatment-related adverse events associated with Alk inhibitors.Curr Oncol. 2014 Feb;21(1):19-26. doi: 10.3747/co.21.1740. Curr Oncol. 2014. PMID: 24523601 Free PMC article.
-
Treating patients with ALK-positive non-small cell lung cancer: latest evidence and management strategy.Ther Adv Med Oncol. 2015 Sep;7(5):274-90. doi: 10.1177/1758834015590593. Ther Adv Med Oncol. 2015. PMID: 26327925 Free PMC article. Review.
References
-
- Shaw AT, Camidge DR, Engelman JA, et al. Clinical activity of crizotinib in advanced non-small cell lung cancer (NSCLC) harboring ROS1 gene rearrangement. ASCO Meeting Abstracts; May 30, 2012; 2012. p. 7508.
-
- Ou S-HI, Kwak EL, Siwak-Tapp C, et al. Activity of Crizotinib (PF02341066), a Dual Mesenchymal-Epithelial Transition (MET) and Anaplastic Lymphoma Kinase (ALK) Inhibitor, in a Non-small Cell Lung Cancer Patient with De Novo MET Amplification. Journal of Thoracic Oncology. 2011;6(5):942–946. 910.1097/JTO.1090b1013e31821528d31821523. - PubMed
-
- Shaw AT, Kim DW, Nagagawa K, Camidge DR, Solomon B. Phase III study of Crizotinib vs. Pemetrexed or Docetaxel Chemotherapy in Patients with Advanced ALK-Positive NSCLC (PROFILE 1007) ESMO. 2012;(2862)
-
- Kim D-W, Ahn M-J, Shi Y, et al. Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC). ASCO Meeting Abstracts; May 30, 2012; 2012. p. 7533.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

