Simultaneous determination of EDTA, sorbic acid, and diclofenac sodium in pharmaceutical preparations using high-performance liquid chromatography
- PMID: 23585291
- PMCID: PMC3666002
- DOI: 10.1208/s12249-013-9962-0
Simultaneous determination of EDTA, sorbic acid, and diclofenac sodium in pharmaceutical preparations using high-performance liquid chromatography
Abstract
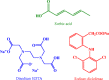
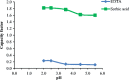
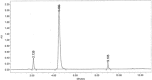
A simple high-performance liquid chromatographic method for simultaneous determination of ethylenediaminetetraacetic acid (EDTA), sorbic acid, and diclofenac sodium was developed and validated. Separation was achieved on a C(18) column (10 cm×4.6 mm) using gradient elution. The mobile phase consisted of acetonitrile-ammonium dihydrogen phosphate buffer solution (0.01 M, pH=2.5, containing 0.8% tetra-n-butyl ammonium hydroxide). The detector wavelength was set at 254 nm. Under these conditions, separation of three compounds was achieved in less than 10 min. The effect of two metal salts and metal concentration on peak area of EDTA was investigated. The pH effect on retention of EDTA and sorbic acid was studied. The method showed linearity for EDTA, sorbic acid, and diclofenac in the ranges of 2.5-100.0, 5.0-200.0, and 20.0-120.0 μg/mL, respectively. The within- and between-day relative standard deviations ranged from 0.52 to 1.94%, 0.50 to 1.34%, and 0.78 to 1.67% for EDTA, sorbic acid, and diclofenac, respectively. The recovery of EDTA, sorbic acid, and diclofenac from pharmaceutical preparation ranged from 96.0-102.0%, 99.7-101.5%, to 97.0-102.5%, respectively. To the best of our knowledge, this is the first report about simultaneous determination of EDTA, sorbic acid, and diclofenac.
Figures


References
-
- Rowe RC, Sheskey PJ, Weller PJ. Handbook of pharmaceutical excipients. 4. London: Science and Practice; 2003. p. 225.
-
- Christianah MA, Li PK, Florey K. Analytical profiles of drug substances. NY: Academic; 1998. pp. 123–140.
-
- Dale M, Parfit K. The complete drug reference. 32. UK: Pharmaceutical Press; 1999.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

