GM-CSF contributes to aortic aneurysms resulting from SMAD3 deficiency
- PMID: 23585475
- PMCID: PMC3635740
- DOI: 10.1172/JCI67356
GM-CSF contributes to aortic aneurysms resulting from SMAD3 deficiency
Abstract
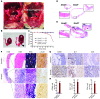
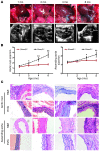
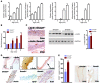
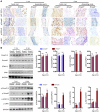
Heterozygous loss-of-function SMAD3 (Mothers against decapentaplegic homolog 3) mutations lead to aneurysm-osteoarthritis syndrome (AOS). In the present study, we found that mice lacking Smad3 had a vascular phenotype similar to AOS, marked by the progressive development of aneurysms. These aneurysms were associated with various pathological changes in transmural inflammatory cell infiltration. Bone marrow transplants from Smad3-/- mice induced aortitis and aortic root dilation in irradiated WT recipient mice. Transplantation of CD4+ T cells from Smad3-/- mice also induced aortitis in Smad3+/+ recipient mice, while depletion of CD4+ T cells in Smad3-/- mice reduced the infiltration of inflammatory cells in the aortic root. Furthermore, IFN-γ deficiency increased, while IL-17 deficiency decreased, disease severity in Smad3+/- mice. Cytokine secretion was measured using a cytokine quantibody array, and Smad3-/- CD4+ T cells secreted more GM-CSF than Smad3+/+ CD4+ T cells. GM-CSF induced CD11b+Gr-1+Ly-6Chi inflammatory monocyte accumulation in the aortic root, but administration of anti-GM-CSF mAb to Smad3-/- mice resulted in significantly less inflammation and dilation in the aortic root. We also identified a missense mutation (c.985A>G) in a family of thoracic aortic aneurysms. Intense inflammatory infiltration and GM-CSF expression was observed in aortas specimens of these patients, suggesting that GM-CSF is potentially involved in the development of AOS.
Figures


References
-
- Dasouki M, et al. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am J Med Genet A. 2007;143A(22):2635–2641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

