A multivariate analysis of the relationship between response and survival among patients with higher-risk myelodysplastic syndromes treated within azacitidine or conventional care regimens in the randomized AZA-001 trial
- PMID: 23585522
- PMCID: PMC3696610
- DOI: 10.3324/haematol.2012.074831
A multivariate analysis of the relationship between response and survival among patients with higher-risk myelodysplastic syndromes treated within azacitidine or conventional care regimens in the randomized AZA-001 trial
Abstract
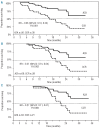
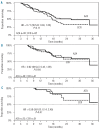
The phase III AZA-001 study established that azacitidine significantly improves overall survival compared with conventional care regimens (hazard ratio 0.58 [95% confidence interval 0.43-0.77], P<0.001). This analysis was conducted to investigate the relationship between treatment response and overall survival. AZA-001 data were analyzed in a multivariate Cox regression analysis with response as a time-varying covariate. Response categories were "Overall Response" (defined as complete remission, partial remission, or any hematologic improvement) and "Stable Disease" (no complete or partial remission, hematologic improvement, or progression) or "Other" (e.g. disease progression). Achieving an Overall Response with azacitidine reduced risk of death by 95% compared with achieving an Overall Response with the conventional care regimens (hazard ratio 0.05 [95%CI: 0.01-0.43], P=0.006). Sensitivity analyses indicated that significantly improved overall survival remained manifest for patients with a hematologic improvement who had never achieved complete or partial remission (hazard ratio 0.19 [95%CI: 0.08-0.46], P<0.001). Stable Disease in both azacitidine-treated and conventional care-treated patients was also associated with a significantly reduced risk of death (hazard ratio 0.09, [95%CI: 0.06-0.15]; P<0.001). These results demonstrate azacitidine benefit on overall survival compared with conventional care regimens in patients with higher-risk myelodysplastic syndromes who achieve hematologic response but never attain complete or partial remission, in addition to the survival advantage conferred by achievement of complete or partial remission.
Trial registration: ClinicalTrials.gov NCT00071799.
Figures

References
-
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997. (6);89:2079–88 - PubMed
-
- Fukumoto JS, Greenberg PL. Management of patients with higher risk myelodysplastic syndromes. Crit Rev Oncol Hematol. 2005;56:(2)179–92 - PubMed
-
- Gore SD, Hermes-DeSantis ER. Enhancing survival outcomes in the management of patients with higher-risk myelodysplastic syndromes. Cancer Control. 2009;16 Suppl:2–10 - PubMed
-
- Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25 - PubMed
-
- Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000; 96(12):3671–4 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

