Analysis of novel iron-regulated, surface-anchored hemin-binding proteins in Corynebacterium diphtheriae
- PMID: 23585541
- PMCID: PMC3697262
- DOI: 10.1128/JB.00244-13
Analysis of novel iron-regulated, surface-anchored hemin-binding proteins in Corynebacterium diphtheriae
Abstract
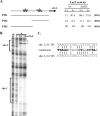
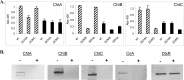
Corynebacterium diphtheriae utilizes hemin and hemoglobin (Hb) as iron sources during growth in iron-depleted environments, and recent studies have shown that the surface-exposed HtaA protein binds both hemin and Hb and also contributes to the utilization of hemin iron. Conserved (CR) domains within HtaA and in the associated hemin-binding protein, HtaB, are required for the ability to bind hemin and Hb. In this study, we identified and characterized two novel genetic loci in C. diphtheriae that encode factors that bind hemin and Hb. Both genetic systems contain two-gene operons that are transcriptionally regulated by DtxR and iron. The gene products of these operons are ChtA-ChtB and ChtC-CirA (previously DIP0522-DIP0523). The chtA and chtB genes are carried on a putative composite transposon associated with C. diphtheriae isolates that dominated the diphtheria outbreak in the former Soviet Union in the 1990s. ChtA and ChtC each contain a single N-terminal CR domain and exhibit significant sequence similarity to each other but only limited similarity with HtaA. The chtB and htaB gene products exhibited a high level of sequence similarity throughout their sequences, and both proteins contain a single CR domain. Whole-cell binding studies as well as protease analysis indicated that all four of the proteins encoded by these two operons are surface exposed, which is consistent with the presence of a transmembrane segment in their C-terminal regions. ChtA, ChtB, and ChtC are able to bind hemin and Hb, with ChtA showing the highest affinity. Site-directed mutagenesis showed that specific tyrosine residues within the ChtA CR domain were critical for hemin and Hb binding. Hemin iron utilization assays using various C. diphtheriae mutants indicate that deletion of the chtA-chtB region and the chtC gene has no affect on the ability of C. diphtheriae to use hemin or Hb as iron sources; however, a chtB htaB double mutant exhibits a significant decrease in hemin iron use, indicating a role in hemin transport for HtaB and ChtB.
Figures






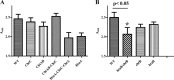
References
-
- Collier RJ. 2001. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon 39:1793–1803 - PubMed
-
- Cornelis P, Andrews SC. 2010. Iron uptake and homeostasis in microorganisms. Caister Academic Press, Norfolk, United Kingdom
-
- Crosa JH, Mey AR, Payne SM. 2004. Iron transport in bacteria. ASM Press, Washington DC
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

