Intracellular translocation and differential accumulation of cell-penetrating peptides in bovine spermatozoa: evaluation of efficient delivery vectors that do not compromise human sperm motility
- PMID: 23585561
- PMCID: PMC3685333
- DOI: 10.1093/humrep/det064
Intracellular translocation and differential accumulation of cell-penetrating peptides in bovine spermatozoa: evaluation of efficient delivery vectors that do not compromise human sperm motility
Abstract
Study question: Do cell penetrating peptides (CPPs) translocate into spermatozoa and, if so, could they be utilized to deliver a much larger protein cargo?
Summary answer: Chemically diverse polycationic CPPs rapidly and efficiently translocate into spermatozoa. They exhibit differential accumulation within intracellular compartments without detrimental influences upon cellular viability or motility but they are relatively ineffective in transporting larger proteins.
What is already known: Endocytosis, the prevalent route of protein internalization into eukaryotic cells, is severely compromised in mature spermatozoa. Thus, the translocation of many bioactive agents into sperm is relatively inefficient. However, the delivery of bioactive moieties into mature spermatozoa could be significantly improved by the identification and utility of an efficient and inert vectorial delivery technology.
Study design: CPP translocation efficacies, their subsequent differential intracellular distribution and the influence of peptides upon viability were determined in bovine spermatozoa. Temporal analyses of sperm motility in the presence of exogenously CPPs utilized normozoospermic human donor samples.
Materials and methods: CPPs were prepared by manual, automated and microwave-enhanced solid phase synthesis. Confocal fluorescence microscopy determined the intracellular distribution of rhodamine-conjugated CPPs in spermatozoa. Quantitative uptake and kinetic analyses compared the translocation efficacies of chemically diverse CPPs and conjugates of biotinylated CPPs and avidin. 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) conversion assays were employed to analyse the influence of CPPs upon sperm cell viability and sperm class assays determined the impact of CPPs on motility in capacitated and non-capacitated human samples.
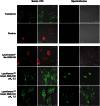
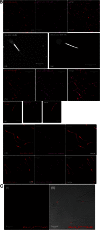
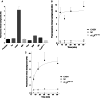
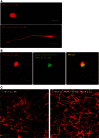
Main results: Chemically heterogeneous CPPs readily translocated into sperm to accumulate within discrete intracellular compartments. Mitoparan (INLKKLAKL(Aib)KKIL), for example, specifically accumulated within the mitochondria located in the sperm midpiece. The unique plasma membrane composition of sperm is a critical factor that directly influences the uptake efficacy of structurally diverse CPPs. No correlations in efficacies were observed when comparing CPP uptake into sperm with either uptake into fibroblasts or direct translocation across a phosphatidylcholine membrane. These comparative investigations identified C105Y (CSIPPEVKFNKPFVYLI) as a most efficient pharmacokinetic modifier for general applications in sperm biology. Significantly, CPP uptake induced no detrimental influence upon either bovine sperm viability or the motility of human sperm. As a consequence of the lack of endocytotic machinery, the CPP-mediated delivery of much larger protein complexes into sperm is relatively inefficient when compared with the similar process in fibroblasts.
Limitations, reasons for caution: It is possible that some CPPs could directly influence aspects of sperm biology and physiology that were not analysed in this study.
Wider implications of the findings: CPP technologies have significant potential to deliver selected bioactive moieties and so could modulate the biology and physiology of human sperm biology both prior- and post-fertilization.
Keywords: cell-penetrating peptide; cytotoxicity; membrane translocation; mitochondrion; motility; spermatozoa.
Figures



Similar articles
-
Protein Mimicry and the Design of Bioactive Cell-Penetrating Peptides: The Genesis of STOPSPERM Bioportides.Methods Mol Biol. 2022;2383:293-306. doi: 10.1007/978-1-0716-1752-6_20. Methods Mol Biol. 2022. PMID: 34766298
-
Genetic, cellular, and structural characterization of the membrane potential-dependent cell-penetrating peptide translocation pore.Elife. 2021 Oct 29;10:e69832. doi: 10.7554/eLife.69832. Elife. 2021. PMID: 34713805 Free PMC article.
-
In Vitro Assays: Friends or Foes of Cell-Penetrating Peptides.Int J Mol Sci. 2020 Jul 2;21(13):4719. doi: 10.3390/ijms21134719. Int J Mol Sci. 2020. PMID: 32630650 Free PMC article. Review.
-
Cell penetration: scope and limitations by the application of cell-penetrating peptides.J Pept Sci. 2014 Oct;20(10):760-84. doi: 10.1002/psc.2672. Epub 2014 Aug 11. J Pept Sci. 2014. PMID: 25112216 Review.
-
Bioportide: an emergent concept of bioactive cell-penetrating peptides.Cell Mol Life Sci. 2012 Sep;69(17):2951-66. doi: 10.1007/s00018-012-0979-4. Epub 2012 Apr 20. Cell Mol Life Sci. 2012. PMID: 22527714 Free PMC article.
Cited by
-
Protein Transduction Domain-Mediated Delivery of Recombinant Proteins and In Vitro Transcribed mRNAs for Protein Replacement Therapy of Human Severe Genetic Mitochondrial Disorders: The Case of Sco2 Deficiency.Pharmaceutics. 2023 Jan 14;15(1):286. doi: 10.3390/pharmaceutics15010286. Pharmaceutics. 2023. PMID: 36678915 Free PMC article. Review.
-
Development of a novel PTD-mediated IVT-mRNA delivery platform for potential protein replacement therapy of metabolic/genetic disorders.Mol Ther Nucleic Acids. 2021 Sep 20;26:694-710. doi: 10.1016/j.omtn.2021.09.008. eCollection 2021 Dec 3. Mol Ther Nucleic Acids. 2021. PMID: 34703653 Free PMC article.
-
Cell-penetrating peptides, targeting the regulation of store-operated channels, slow decay of the progesterone-induced [Ca2+]i signal in human sperm.Mol Hum Reprod. 2015 Jul;21(7):563-70. doi: 10.1093/molehr/gav019. Epub 2015 Apr 16. Mol Hum Reprod. 2015. PMID: 25882543 Free PMC article.
-
Exosome Composition and Seminal Plasma Proteome: A Promising Source of Biomarkers of Male Infertility.Int J Mol Sci. 2020 Sep 24;21(19):7022. doi: 10.3390/ijms21197022. Int J Mol Sci. 2020. PMID: 32987677 Free PMC article. Review.
-
Perfect date-the review of current research into molecular bases of mammalian fertilization.J Assist Reprod Genet. 2020 Feb;37(2):243-256. doi: 10.1007/s10815-019-01679-4. Epub 2020 Jan 6. J Assist Reprod Genet. 2020. PMID: 31909446 Free PMC article. Review.
References
-
- Abou-haila A, Tulsiani DRP. Signal transduction pathways that regulate sperm capacitation and the acrosome reaction. Arch Biochem Biophys. 2009;485:72–81. - PubMed
-
- Bedu-Addo K, Lefièvre L, Mosely FLC, Barratt CLR, Publicover SJ. Bicarbonate and bovine serum albumin reversibly ‘switch’ capacitation-induced events in human spermatozoa. Mol Hum Reprod. 2005;11:683–691. - PubMed
-
- Boerke A, Dielman SJ, Gadella BM. A possible role for sperm RNA in early embryo development. Theriogenology. 2007;68S:S147–S155. - PubMed
-
- Castro-González D, Alvarez M, Muro J, Esteso MC, de Paz P, Anel L, Martínez-Pastor F. The acidic probe LysoSensor is not useful for acrosome evaluation of cryopreserved ram spermatozoa. Reprod Domest Anim. 2010;45:363–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

