ACYL-LIPID DESATURASE2 is required for chilling and freezing tolerance in Arabidopsis
- PMID: 23585650
- PMCID: PMC3663278
- DOI: 10.1105/tpc.113.111179
ACYL-LIPID DESATURASE2 is required for chilling and freezing tolerance in Arabidopsis
Abstract
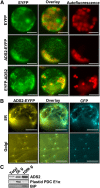
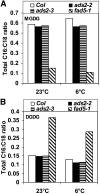
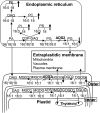
Fatty acid desaturation of membrane lipids is a strategy for plants to survive chilling or freezing temperature. To further characterize enzymes involved in this stress response pathway, ACYL-LIPID DESATURASE2 (ADS2; Enzyme Commission 1.14.99) was studied using genetic, cell, and biochemical approaches. ads2 mutant plants appear similar to the wild type under standard growth conditions but display a dwarf and sterile phenotype when grown at 6°C and also show increased sensitivity to freezing temperature. Fatty acid composition analysis demonstrated that ads2 mutant plants at 6°C have reduced levels of 16:1, 16:2, 16:3, and 18:3 and higher levels of 16:0 and 18:0 fatty acids compared with the wild type. Lipid profiling revealed that 34C species of phosphatidylglycerol (PG) and monogalactosyl diacylglycerol (MGDG) content in ads2 mutants were lower and phosphatidic acid, phosphatidylinositol, phosphatidylethanolamine, phosphatidylcholine, lyso-phosphatidylcholine, and phosphatidylserine were higher than the wild type. Subcellular localization of C- and N-terminal enhanced fluorescence fusion proteins indicated that ADS2 localized primarily to the endoplasmic reticulum, although signal was also confirmed in Golgi and plastids. A double mutation with a putative plastid ADS3 paralog exacerbates the growth defects of ads2 mutant plants under low temperature. These observations suggest that ADS2 encodes a 16:0 desaturase of MGDG and PG. We hypothesize that a low temperature-induced shift from the plastid to endoplasmic reticulum pathway for membrane lipid biosynthesis is required for the cold stress response in Arabidopsis thaliana, and ADS2 is essential to adjust the acyl composition of organelle membrane lipid composition in response to cold stress.
Figures







References
-
- Andersson M.X., Larsson K.E., Tjellström H., Liljenberg C., Sandelius A.S. (2005). Phosphate-limited oat. The plasma membrane and the tonoplast as major targets for phospholipid-to-glycolipid replacement and stimulation of phospholipases in the plasma membrane. J. Biol. Chem. 280: 27578–27586 - PubMed
-
- Andersson M.X., Stridh M.H., Larsson K.E., Liljenberg C., Sandelius A.S. (2003). Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett. 537: 128–132 - PubMed
-
- Awai K., Maréchal E., Block M.A., Brun D., Masuda T., Shimada H., Takamiya K., Ohta H., Joyard J. (2001). Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 98: 10960–10965 - PMC - PubMed
-
- Bao X., Thelen J.J., Bonaventure G., Ohlrogge J.B. (2003). Characterization of cyclopropane fatty-acid synthase from Sterculia foetida. J. Biol. Chem. 278: 12846–12853 - PubMed
-
- Browse J., McCourt P., Somerville C.R. (1985). A mutant of Arabidopsis lacking a chloroplast-specific lipid. Science 227: 763–765 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

