Arabidopsis 3-ketoacyl-coenzyme a synthase9 is involved in the synthesis of tetracosanoic acids as precursors of cuticular waxes, suberins, sphingolipids, and phospholipids
- PMID: 23585652
- PMCID: PMC3668053
- DOI: 10.1104/pp.112.210450
Arabidopsis 3-ketoacyl-coenzyme a synthase9 is involved in the synthesis of tetracosanoic acids as precursors of cuticular waxes, suberins, sphingolipids, and phospholipids
Abstract
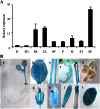
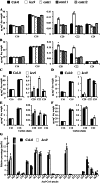
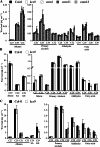
Very-long-chain fatty acids (VLCFAs) with chain lengths from 20 to 34 carbons are involved in diverse biological functions such as membrane constituents, a surface barrier, and seed storage compounds. The first step in VLCFA biosynthesis is the condensation of two carbons to an acyl-coenzyme A, which is catalyzed by 3-ketoacyl-coenzyme A synthase (KCS). In this study, amino acid sequence homology and the messenger RNA expression patterns of 21 Arabidopsis (Arabidopsis thaliana) KCSs were compared. The in planta role of the KCS9 gene, showing higher expression in stem epidermal peels than in stems, was further investigated. The KCS9 gene was ubiquitously expressed in various organs and tissues, including roots, leaves, and stems, including epidermis, silique walls, sepals, the upper portion of the styles, and seed coats, but not in developing embryos. The fluorescent signals of the KCS9::enhanced yellow fluorescent protein construct were merged with those of BrFAD2::monomeric red fluorescent protein, which is an endoplasmic reticulum marker in tobacco (Nicotiana benthamiana) epidermal cells. The kcs9 knockout mutants exhibited a significant reduction in C24 VLCFAs but an accumulation of C20 and C22 VLCFAs in the analysis of membrane and surface lipids. The mutant phenotypes were rescued by the expression of KCS9 under the control of the cauliflower mosaic virus 35S promoter. Taken together, these data demonstrate that KCS9 is involved in the elongation of C22 to C24 fatty acids, which are essential precursors for the biosynthesis of cuticular waxes, aliphatic suberins, and membrane lipids, including sphingolipids and phospholipids. Finally, possible roles of unidentified KCSs are discussed by combining genetic study results and gene expression data from multiple Arabidopsis KCSs.
Figures





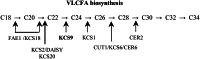
References
-
- Aarts MG, Hodge R, Kalantidis K, Florack D, Wilson ZA, Mulligan BJ, Stiekema WJ, Scott R, Pereira A. (1997) The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J 12: 615–623 - PubMed
-
- An G. (1987) Binary Ti vectors for plant transformation and promoter analysis. Methods Enzymol 153: 292–305
-
- Barthlott W, Neinhuis C. (1997) Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann Bot (Lond) 79: 667–677
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

