Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid
- PMID: 23585680
- PMCID: PMC3647383
- DOI: 10.4049/jimmunol.1300097
Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid
Abstract
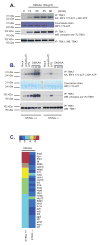
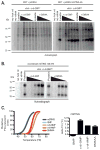
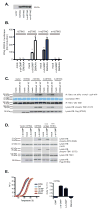
Vascular disrupting agents such as 5,6-dimethylxanthenone-4-acetic acid (DMXAA) represent a novel approach for cancer treatment. DMXAA has potent antitumor activity in mice and, despite significant preclinical promise, failed human clinical trials. The antitumor activity of DMXAA has been linked to its ability to induce type I IFNs in macrophages, although the molecular mechanisms involved are poorly understood. In this study, we identify stimulator of IFN gene (STING) as a direct receptor for DMXAA leading to TANK-binding kinase 1 and IFN regulatory factor 3 signaling. Remarkably, the ability to sense DMXAA was restricted to murine STING. Human STING failed to bind to or signal in response to DMXAA. Human STING also failed to signal in response to cyclic dinucleotides, conserved bacterial second messengers known to bind and activate murine STING signaling. Collectively, these findings detail an unexpected species-specific role for STING as a receptor for an anticancer drug and uncover important insights that may explain the failure of DMXAA in clinical trials for human cancer.
Figures


References
-
- Rakoff-Nahoum S, Medzhitov R. Role of toll-like receptors in tissue repair and tumorigenesis. Biochemistry Biokhimiia. 2008;73:555–561. - PubMed
-
- Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nature reviews Cancer. 2009;9:57–63. - PubMed
-
- Baguley BC, Ching LM. Immunomodulatory actions of xanthenone anticancer agents. BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. 1997;8:119–127. - PubMed
-
- Wallace A, LaRosa DF, Kapoor V, Sun J, Cheng G, Jassar A, Blouin A, Ching LM, Albelda SM. The vascular disrupting agent, DMXAA, directly activates dendritic cells through a MyD88-independent mechanism and generates antitumor cytotoxic T lymphocytes. Cancer research. 2007;67:7011–7019. - PubMed
-
- Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching LM, Kaiser LR, Albelda SM. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer research. 2005;65:11752–11761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI067497/AI/NIAID NIH HHS/United States
- AI080749/AI/NIAID NIH HHS/United States
- R56 AI067497/AI/NIAID NIH HHS/United States
- AI067497/AI/NIAID NIH HHS/United States
- R01 AI093752/AI/NIAID NIH HHS/United States
- R01 AI018797/AI/NIAID NIH HHS/United States
- P01 AI063302/AI/NIAID NIH HHS/United States
- R01 AI080749/AI/NIAID NIH HHS/United States
- T32 AI095213/AI/NIAID NIH HHS/United States
- F32 AI091100/AI/NIAID NIH HHS/United States
- R37 AI067497/AI/NIAID NIH HHS/United States
- R21 AI103817/AI/NIAID NIH HHS/United States
- AI091100/AI/NIAID NIH HHS/United States
- AI063302/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

