Local "on-demand" generation and function of antigen-specific Foxp3+ regulatory T cells
- PMID: 23585681
- PMCID: PMC3646944
- DOI: 10.4049/jimmunol.1202625
Local "on-demand" generation and function of antigen-specific Foxp3+ regulatory T cells
Abstract
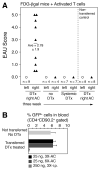
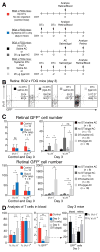
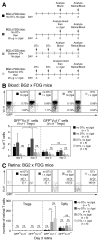
Extrathymically derived regulatory T cells (iTregs) protect against autoimmunity to tissue-specific Ags. However, whether Ag-specific iTreg generation and function is limited to secondary lymphoid tissue or whether it can occur within the tissue-specific local environment of the cognate Ag remains unresolved. Mice expressing β-galactosidase (βgal) on a retina-specific promoter (βgal mice) in conjunction with mice expressing GFP and diphtheria toxin (DTx) receptor (DTR) under control of the Foxp3 promoter, and βgal-specific TCR transgenic (BG2) mice were used to examine this question. Local depletion (ocular DTx), but not systemic depletion (i.p. DTx), of βgal-specific iTregs enhanced experimental autoimmune uveoretinitis induced by activated βgal-specific effector T cells. Injections of small amounts of βgal into the anterior chamber of the eye produced similar numbers of βgal-specific iTregs in the retina whether the mouse was depleted of pre-existing, circulating Tregs. Taken together, these results suggest that protection from tissue-specific autoimmunity depends on the function of local Ag-specific iTregs and that the retina is capable of local, "on-demand" iTreg generation that is independent of circulating Tregs.
Figures






References
-
- Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:19–201. - PubMed
-
- Jutel M, Akdis CA. T-cell regulatory mechanisms in specific immunotherapy. Chem Immunol Allergy. 2008;94:158–177. - PubMed
-
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. - PubMed
-
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins S, Turley SJ, Von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self-shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. - PubMed
-
- Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

