Effect of iron chelators on methemoglobin and thrombin preconditioning
- PMID: 23585819
- PMCID: PMC3623273
- DOI: 10.1007/s12975-012-0195-4
Effect of iron chelators on methemoglobin and thrombin preconditioning
Abstract
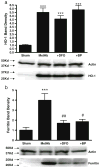
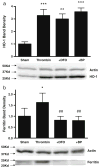
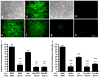
Cell loss immediately adjacent to an intracerebral hemorrhage may be mediated in part by the toxicities of extracellular hemoglobin (Hb) and thrombin. However, at low concentrations, these proteins induce tolerance to hemin and iron that may limit further peri-hematomal injury as erythrocyte lysis progresses. The mechanisms mediating these preconditioning effects have not been completely defined, but increased expression of both heme oxygenase (HO)-1 and iron binding proteins likely contributes. In the present study, we hypothesized that iron chelator therapy would attenuate this protective response. Pretreatment of cortical glial cultures (> 90 % GFAP+) with 3 μM methemoglobin (metHb) or 5 units/ml thrombin for 24 h was nontoxic per se, and increased HO-1 and ferritin expression. When challenged with a toxic concentration of hemin, the increase in cellular redox-active iron was attenuated in preconditioned cultures and cell survival was increased. However, if cultures were pretreated with metHb or thrombin plus deferoxamine or 2,2'-bipyridyl, ferritin induction was prevented and cellular redox-active iron increased with hemin treatment. Preconditioning-mediated cytoprotection was consistently reduced by deferoxamine, while 2,2'-bipyridyl had a variable effect. Neither chelator altered HO-1 expression. A cytoprotective response was preserved when chelator therapy was limited to 11 hours of the 24 h preconditioning interval. These results suggest a potentially deleterious effect of continuous iron chelator therapy after ICH. Intermittent therapy may remove peri-hematomal iron without negating the benefits of exposure to low concentrations of Hb or thrombin.
Keywords: Heme; Intracerebral hemorrhage; Iron; Ischemia; Stroke; Subarachnoid hemorrhage.
Figures


References
-
- Ardizzone TD, Zhan X, Ander BP, Sharp FR. SRC kinase inhibition improves acute outcomes after experimental intracerebral hemorrhage. Stroke. 2007;38(5):1621–5. - PubMed
-
- Gong Y, Xi GH, Keep RF, Hoff JT, Hua Y. Complement inhibition attenuates brain edema and neurological deficits induced by thrombin. Acta Neurochir Suppl. 2005;95:389–92. - PubMed
-
- Alyash A. Redox and radical reactions of hemoglobin solutions: toxicities and protective strategies. In: Winslow R, editor. Blood Substitutes. London: Academic Press; 2006. pp. 197–205.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

