Mutation of the fiber shaft heparan sulphate binding site of a 5/3 chimeric adenovirus reduces liver tropism
- PMID: 23585829
- PMCID: PMC3621953
- DOI: 10.1371/journal.pone.0060032
Mutation of the fiber shaft heparan sulphate binding site of a 5/3 chimeric adenovirus reduces liver tropism
Abstract
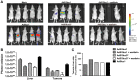
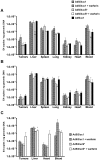
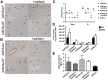
Natural tropism to the liver is a major obstacle in systemic delivery of adenoviruses in cancer gene therapy. Adenovirus binding to soluble coagulation factors and to cellular heparan sulphate proteoglycans via the fiber shaft KKTK domain are suggested to cause liver tropism. Serotype 5 adenovirus constructs with mutated KKTK regions exhibit liver detargeting, but they also transduce tumors less efficiently, possibly due to altered fiber conformation. We constructed Ad5/3lucS*, a 5/3 chimeric adenovirus with a mutated KKTK region. The fiber knob swap was hypothesized to facilitate tumor transduction. This construct was studied with or without additional coagulation factor ablation. Ad5/3lucS* exhibited significantly reduced transduction of human hepatic cells in vitro and mouse livers in vivo. Combination of coagulation factor ablation by warfarinization to Ad5/3lucS* seemed to further enhance liver detargeting. Cancer cell transduction by Ad5/3lucS* was retained in vitro. In vivo, viral particle accumulation in M4A4-LM3 xenograft tumors was comparable to controls, but Ad5/3lucS* transgene expression was nearly abolished. Coagulation factor ablation did not affect tumor transduction. These studies set the stage for further investigations into the effects of the KKTK mutation and coagulation factor ablation in the context of 5/3 serotype chimerism. Of note, the putative disconnect between tumor transduction and transgene expression could prove useful in further understanding of adenovirus biology.
Conflict of interest statement
Figures





References
-
- Fukuhara H, Homma Y, Todo T (2009) Oncolytic virus therapy for prostate cancer. Int J Urol 17: 20–30. - PubMed
-
- Liang M (2011) Clinical Development of Oncolytic viruses in China. Curr Pharm Biotechnol. - PubMed
-
- Nemunaitis J, Senzer N, Sarmiento S, Zhang YA, Arzaga R, et al. (2007) A phase I trial of intravenous infusion of ONYX-015 and enbrel in solid tumor patients. Cancer Gene Ther 14: 885–893. - PubMed
-
- Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, et al. (2006) A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther 14: 107–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

