Oncomir miR-125b suppresses p14(ARF) to modulate p53-dependent and p53-independent apoptosis in prostate cancer
- PMID: 23585871
- PMCID: PMC3621663
- DOI: 10.1371/journal.pone.0061064
Oncomir miR-125b suppresses p14(ARF) to modulate p53-dependent and p53-independent apoptosis in prostate cancer
Abstract
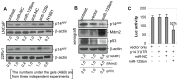
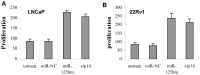
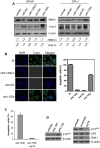
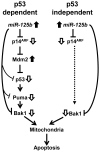
MicroRNAs are a class of naturally occurring small non-coding RNAs that target protein-coding mRNAs at the post-transcriptional level and regulate complex patterns of gene expression. Our previous studies demonstrated that in human prostate cancer the miRNA miR-125b is highly expressed, leading to a negative regulation of some tumor suppressor genes. In this study, we further extend our studies by showing that miR-125b represses the protein product of the ink4a/ARF locus, p14(ARF), in two prostate cancer cell lines, LNCaP (wild type-p53) and 22Rv1 (both wild type and mutant p53), as well as in the PC-346C prostate cancer xenograft model that lentivirally overexpressed miR-125b. Our results highlight that miR-125b modulates the p53 network by hindering the down-regulation of Mdm2, thereby affecting p53 and its target genes p21 and Puma to a degree sufficient to inhibit apoptosis. Conversely, treatment of prostate cancer cells with an inhibitor of miR-125b (anti-miR-125b) resulted in increased expression of p14(ARF), decreased level of Mdm2, and induction of apoptosis. In addition, overexpression of miR-125b in p53-deficient PC3 cells induced down-regulation of p14(ARF), which leads to increased cell proliferation through a p53-independent manner. Thus, we conclude that miR-125b acts as an oncogene which regulates p14(ARF)/Mdm2 signaling, stimulating proliferation of prostate cancer cells through a p53-dependent or p53-independent function. This reinforces our belief that miR-125b has potential as a therapeutic target for the management of patients with metastatic prostate cancer.
Conflict of interest statement
Figures


References
-
- Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA: a cancer journal for clinicians 62: 10–29. - PubMed
-
- Lassi K, Dawson NA (2009) Emerging therapies in castrate-resistant prostate cancer. Current opinion in oncology 21: 260–265. - PubMed
-
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

