Requirement of heterogeneous nuclear ribonucleoprotein C for BRCA gene expression and homologous recombination
- PMID: 23585894
- PMCID: PMC3621867
- DOI: 10.1371/journal.pone.0061368
Requirement of heterogeneous nuclear ribonucleoprotein C for BRCA gene expression and homologous recombination
Abstract
Background: Heterogeneous nuclear ribonucleoprotein C1/C2 (hnRNP C) is a core component of 40S ribonucleoprotein particles that bind pre-mRNAs and influence their processing, stability and export. Breast cancer tumor suppressors BRCA1, BRCA2 and PALB2 form a complex and play key roles in homologous recombination (HR), DNA double strand break (DSB) repair and cell cycle regulation following DNA damage.
Methods: PALB2 nucleoprotein complexes were isolated using tandem affinity purification from nuclease-solubilized nuclear fraction. Immunofluorescence was used for localization studies of proteins. siRNA-mediated gene silencing and flow cytometry were used for studying DNA repair efficiency and cell cycle distribution/checkpoints. The effect of hnRNP C on mRNA abundance was assayed using quantitative reverse transcriptase PCR.
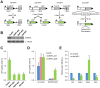
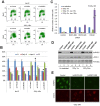
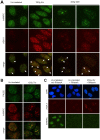
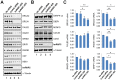
Results and significance: We identified hnRNP C as a component of a nucleoprotein complex containing breast cancer suppressor proteins PALB2, BRCA2 and BRCA1. Notably, other components of the 40S ribonucleoprotein particle were not present in the complex. hnRNP C was found to undergo significant changes of sub-nuclear localization after ionizing radiation (IR) and to partially localize to DNA damage sites. Depletion of hnRNP C substantially altered the normal balance of repair mechanisms following DSB induction, reducing HR usage in particular, and impaired S phase progression after IR. Moreover, loss of hnRNP C strongly reduced the abundance of key HR proteins BRCA1, BRCA2, RAD51 and BRIP1, which can be attributed, at least in part, to the downregulation of their mRNAs due to aberrant splicing. Our results establish hnRNP C as a key regulator of BRCA gene expression and HR-based DNA repair. They also suggest the existence of an RNA regulatory program at sites of DNA damage, which involves a unique function of hnRNP C that is independent of the 40S ribonucleoprotein particles and most other hnRNP proteins.
Conflict of interest statement
Figures


References
-
- Beyer AL, Christensen ME, Walker BW, LeStourgeon WM (1977) Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell 11: 127–138. - PubMed
-
- Gorlach M, Burd CG, Dreyfuss G (1994) The determinants of RNA-binding specificity of the heterogeneous nuclear ribonucleoprotein C proteins. J Biol Chem 269: 23074–23078. - PubMed
-
- McAfee JG, Soltaninassab SR, Lindsay ME, LeStourgeon WM (1996) Proteins C1 and C2 of heterogeneous nuclear ribonucleoprotein complexes bind RNA in a highly cooperative fashion: support for their contiguous deposition on pre-mRNA during transcription. Biochemistry 35: 1212–1222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

