Inflammation and immunity in radiation damage to the gut mucosa
- PMID: 23586015
- PMCID: PMC3614034
- DOI: 10.1155/2013/123241
Inflammation and immunity in radiation damage to the gut mucosa
Abstract
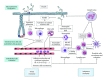
Erythema was observed on the skin of the first patients treated with radiation therapy. It is in particular to reduce this erythema, one feature of tissue inflammation, that prescribed dose to the tumor site started to be fractionated. It is now well known that radiation exposure of normal tissues generates a sustained and apparently uncontrolled inflammatory process. Radiation-induced inflammation is always observed, often described, sometimes partly explained, but still today far from being completely understood. The thing with the gut and especially the gut mucosa is that it is at the frontier between the external milieu and the organism, is in contact with a plethora of commensal and foreign antigens, possesses a dense-associated lymphoid tissue, and is particularly radiation sensitive because of a high mucosal turnover rate. All these characteristics make the gut mucosa a strong responsive organ in terms of radiation-induced immunoinflammation. This paper will focus on what has been observed in the normal gut and what remains to be done concerning the immunoinflammatory response following localized radiation exposure.
Figures

References
-
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex “wound”. Radiotherapy and Oncology. 2002;63(2):129–145. - PubMed
-
- Andreyev HJN, Wotherspoon A, Denham JW, Hauer-Jensen M. “Pelvic radiation disease”: new understanding and new solutions for a new disease in the era of cancer survivorship. Scandinavian Journal of Gastroenterology. 2011;46(4):389–397. - PubMed
-
- Andreyev HJN, Wotherspoon A, Denham JW, Hauer-Jensen M. Defining pelvic-radiation disease for the survivorship era. The Lancet Oncology. 2010;11(4):310–312. - PubMed
-
- Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116(1):7–14. - PubMed
-
- Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nature Reviews Cancer. 2006;6(9):702–713. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

