Early stimulation treatment provides complete sensory-induced protection from ischemic stroke under isoflurane anesthesia
- PMID: 23586641
- PMCID: PMC3735829
- DOI: 10.1111/ejn.12217
Early stimulation treatment provides complete sensory-induced protection from ischemic stroke under isoflurane anesthesia
Abstract
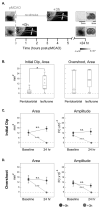
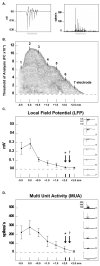
Using a rodent model of ischemia [permanent middle cerebral artery occlusion (pMCAO)], previous studies demonstrated that whisker stimulation treatment completely protects the cortex from impending stroke when initiated within 2 h following pMCAO. When initiated 3 h post-pMCAO, the identical treatment exacerbates stroke damage. Rats in these studies, however, were anesthetised with sodium pentobarbital, whereas human stroke patients are typically awake. To overcome this drawback, our laboratory has begun to use the anesthetic isoflurane, which allows rats to rapidly recover from pMCAO within minutes, to test stimulation treatment in awake rats and to determine whether isoflurane has an effect upon the pMCAO stroke model. We found no difference in infarct volume between pMCAO in untreated controls under either sodium pentobarbital or isoflurane, and the primary finding was that rats that received treatment immediately post-pMCAO maintain cortical function and no stroke damage, whereas rats that received treatment 3 h post-pMCAO exhibited eliminated cortical activity and extensive stroke damage. The only difference between anesthetics was the broad extent of evoked cortical activity observed during both functional imaging and electrophysiological recording, suggesting that the extent of evoked activity evident under isoflurane anesthesia is supported by underlying neuronal activity. Given the high degree of similarity with previous data, we conclude that the pMCAO stroke model is upheld with the use of isoflurane. This study demonstrated that the isoflurane-anesthetised rat pMCAO model can be used for cerebrovascular studies, and allows for highly detailed investigation of potential novel treatments for ischemic stroke using awake, behaving animals.
Keywords: isoflurane; protection; rat; rodent model; stimulation treatment; stroke.
© 2013 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures


References
-
- Alkire MT. Probing the mind: anesthesia and neuroimaging. Clin Pharmacol Ther. 2008;84:149–152. - PubMed
-
- Baughman VL, Hoffman WE, Miletich DJ, Albrecht RF, Thomas C. Neurologic outcome in rats following incomplete cerebral ischemia during halothane, isoflurane, or N2O. Anesthesiology. 1988;69:192–198. - PubMed
-
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. - PubMed
-
- Berger T, Borgdorff A, Crochet S, Neubauer FB, Lefort S, Fauvet B, Ferezou I, Carleton A, Luscher HR, Petersen CC. Combined voltage and calcium epifluorescence imaging in vitro and in vivo reveals subthreshold and suprathreshold dynamics of mouse barrel cortex. J Neurophysiol. 2007;97:3751–3762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

