Pathway specific modulation of S1P1 receptor signalling in rat and human astrocytes
- PMID: 23587004
- PMCID: PMC3696333
- DOI: 10.1111/bph.12207
Pathway specific modulation of S1P1 receptor signalling in rat and human astrocytes
Abstract
Background and purpose: The sphingosine 1-phosphate receptor subtype 1 (S1P1R) is modulated by phosphorylated FTY720 (pFTY720), which causes S1P1R internalization preventing lymphocyte migration thus limiting autoimmune response. Studies indicate that internalized S1P1Rs continue to signal, maintaining an inhibition of cAMP, thus raising question whether the effects of pFTY720 are due to transient initial agonism, functional antagonism and/or continued signalling. To further investigate this, the current study first determined if continued S1P1R activation is pathway specific.
Experimental approach: Using human and rat astrocyte cultures, the effects of S1P1R activation on cAMP, pERK and Ca(2+) signalling was investigated. In addition, to examine the role of S1P1R redistribution on these events, a novel biologic (MNP301) that prevented pFTY720-mediated S1P1R redistribution was engineered.
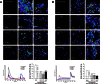
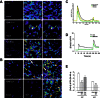
Key results: The data showed that pFTY720 induced long-lasting S1P1R redistribution and continued cAMP signalling in rat astrocytes. In contrast, pFTY720 induced a transient increase of Ca(2+) in astrocytes and subsequent antagonism of Ca(2+) signalling. Notably, while leaving pFTY720-induced cAMP signalling intact, the novel MNP301 peptide attenuated S1P1R-mediated Ca(2+) and pERK signalling in cultured rat astrocytes.
Conclusions and implications: These findings suggested that pFTY720 causes continued cAMP signalling that is not dependent on S1P1R redistribution and induces functional antagonism of Ca(2+) signalling after transient stimulation. To our knowledge, this is the first report demonstrating that pFTY720 causes continued signalling in one pathway (cAMP) versus functional antagonism of another pathway (Ca(2+)) and which also suggests that redistributed S1P1Rs may have differing signalling properties from those expressed at the surface.
© 2013 The British Pharmacological Society.
Figures






References
-
- Bassi R, Anelli V, Giussani P, Tettamanti G, Viani P, Riboni L. Sphingosine-1-phosphate is released by cerebellar astrocytes in response to bFGF and induces astrocyte proliferation through G(i)-protein-coupled receptors. Glia. 2006;53:621–630. - PubMed
-
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimers as well as alpha subunits. Cell. 1992;71:1069–1072. - PubMed
-
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

