Phylostratigraphic profiles reveal a deep evolutionary history of the vertebrate head sensory systems
- PMID: 23587066
- PMCID: PMC3636138
- DOI: 10.1186/1742-9994-10-18
Phylostratigraphic profiles reveal a deep evolutionary history of the vertebrate head sensory systems
Abstract
Background: The vertebrate head is a highly derived trait with a heavy concentration of sophisticated sensory organs that allow complex behaviour in this lineage. The head sensory structures arise during vertebrate development from cranial placodes and the neural crest. It is generally thought that derivatives of these ectodermal embryonic tissues played a central role in the evolutionary transition at the onset of vertebrates. Despite the obvious importance of head sensory organs for vertebrate biology, their evolutionary history is still uncertain.
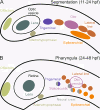
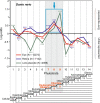
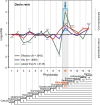
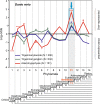
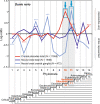
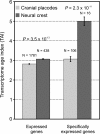
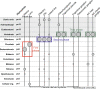
Results: To give a fresh perspective on the adaptive history of the vertebrate head sensory organs, we applied genomic phylostratigraphy to large-scale in situ expression data of the developing zebrafish Danio rerio. Contrary to traditional predictions, we found that dominant adaptive signals in the analyzed sensory structures largely precede the evolutionary advent of vertebrates. The leading adaptive signals at the bilaterian-chordate transition suggested that the visual system was the first sensory structure to evolve. The olfactory, vestibuloauditory, and lateral line sensory organs displayed a strong link with the urochordate-vertebrate ancestor. The only structures that qualified as genuine vertebrate innovations were the neural crest derivatives, trigeminal ganglion and adenohypophysis. We also found evidence that the cranial placodes evolved before the neural crest despite their proposed embryological relatedness.
Conclusions: Taken together, our findings reveal pre-vertebrate roots and a stepwise adaptive history of the vertebrate sensory systems. This study also underscores that large genomic and expression datasets are rich sources of macroevolutionary information that can be recovered by phylostratigraphic mining.
Figures

Similar articles
-
Phylostratigraphic profiles in zebrafish uncover chordate origins of the vertebrate brain.Mol Biol Evol. 2015 Feb;32(2):299-312. doi: 10.1093/molbev/msu319. Epub 2014 Nov 17. Mol Biol Evol. 2015. PMID: 25415965 Free PMC article.
-
Vertebrate cranial placodes as evolutionary innovations--the ancestor's tale.Curr Top Dev Biol. 2015;111:235-300. doi: 10.1016/bs.ctdb.2014.11.008. Epub 2015 Jan 20. Curr Top Dev Biol. 2015. PMID: 25662263 Review.
-
Making a head: Neural crest and ectodermal placodes in cranial sensory development.Semin Cell Dev Biol. 2023 Mar 30;138:15-27. doi: 10.1016/j.semcdb.2022.06.009. Epub 2022 Jun 25. Semin Cell Dev Biol. 2023. PMID: 35760729 Free PMC article. Review.
-
The evolutionary history of vertebrate cranial placodes II. Evolution of ectodermal patterning.Dev Biol. 2014 May 1;389(1):98-119. doi: 10.1016/j.ydbio.2014.01.019. Epub 2014 Feb 1. Dev Biol. 2014. PMID: 24491817 Review.
-
The evolution of sensory placodes.ScientificWorldJournal. 2006 Apr 4;6:1841-50. doi: 10.1100/tsw.2006.314. ScientificWorldJournal. 2006. PMID: 17205191 Free PMC article. Review.
Cited by
-
New Genes Interacted With Recent Whole-Genome Duplicates in the Fast Stem Growth of Bamboos.Mol Biol Evol. 2021 Dec 9;38(12):5752-5768. doi: 10.1093/molbev/msab288. Mol Biol Evol. 2021. PMID: 34581782 Free PMC article.
-
Melanocytes and photosensory organs share a common ancestry that illuminates the origins of the neural crest.Commun Biol. 2025 Jul 23;8(1):1092. doi: 10.1038/s42003-025-08502-0. Commun Biol. 2025. PMID: 40696183 Free PMC article.
-
Heterogeneous molecular processes among the causes of how sequence similarity scores can fail to recapitulate phylogeny.Brief Bioinform. 2017 May 1;18(3):451-457. doi: 10.1093/bib/bbw034. Brief Bioinform. 2017. PMID: 27103098 Free PMC article.
-
Systematic errors in orthology inference and their effects on evolutionary analyses.iScience. 2021 Jan 28;24(2):102110. doi: 10.1016/j.isci.2021.102110. eCollection 2021 Feb 19. iScience. 2021. PMID: 33659875 Free PMC article.
-
Evolutionary Analysis of the Bacillus subtilis Genome Reveals New Genes Involved in Sporulation.Mol Biol Evol. 2020 Jun 1;37(6):1667-1678. doi: 10.1093/molbev/msaa035. Mol Biol Evol. 2020. PMID: 32061128 Free PMC article.
References
-
- Schmidt-Rhaesa A. The Evolution of Organ Systems. 1. USA: Oxford University Press; 2007.
LinkOut - more resources
Full Text Sources
Other Literature Sources

