"Prion-like" templated misfolding in tauopathies
- PMID: 23587140
- PMCID: PMC8028860
- DOI: 10.1111/bpa.12044
"Prion-like" templated misfolding in tauopathies
Abstract
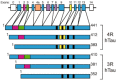
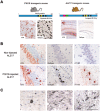
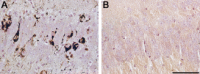
The soluble microtubule-associated protein tau forms hyperphosphorylated, insoluble and filamentous inclusions in a number of neurodegenerative diseases referred to as "tauopathies." In Alzheimer's disease, tau pathology develops in a stereotypical manner, with the first lesions appearing in the locus coeruleus and entorhinal cortex, from where they appear to spread to the hippocampus and neocortex. Propagation of tau pathology is also a characteristic of argyrophilic grain disease, where the tau lesions spread throughout the limbic system. Significantly, isoform composition and morphology of tau filaments can differ between tauopathies, suggesting the existence of distinct tau strains. Extensive experimental findings indicate that prion-like mechanisms underly the pathogenesis of tauopathies.
© 2013 The Authors; Brain Pathology © 2013 International Society of Neuropathology.
Conflict of interest statement
The authors declare that they have no conflict of interest related to this article.
Figures
References
-
- Akoury E, Gajda M, Pickhardt M, Biernat J, Soraya P, Griesinger C et al (2013) Inhibition of tau filament formation by conformational modulation. J Am Chem Soc 35:2853–2862. - PubMed
-
- Andreadis A, Brown WM, Kosik KS (1992) Structure and novel exons of the human tau gene. Biochemistry 31:10626–10633. - PubMed
-
- Arai T, Ikeda K, Akiyama H, Nonaka T, Hasegawa M, Ishiguro K et al (2004) Identification of amino‐terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol 55:72–79. - PubMed
-
- Arnold CS, Johnson GV, Cole RN, Dong DL, Lee M, Hart GW (1996) The microtubule‐associated protein tau is extensively modified with O‐linked N‐acetylglucosamine. J Biol Chem 271:28741–28744. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

