Annexin A1 protein regulates the expression of PMVEC cytoskeletal proteins in CBDL rat serum-induced pulmonary microvascular remodeling
- PMID: 23587191
- PMCID: PMC3641942
- DOI: 10.1186/1479-5876-11-98
Annexin A1 protein regulates the expression of PMVEC cytoskeletal proteins in CBDL rat serum-induced pulmonary microvascular remodeling
Abstract
Background: Hepatopulmonary syndrome (HPS) is characterized by advanced liver disease, hypoxemia and intrapulmonary vascular dilatation (IPVD). The pathogenesis of HPS is not completely understood. Recent findings have established the role of proliferation and phenotype differentiation of pulmonary microvascular endothelial cells (PMVECs) in IPVD of HPS; the change in cytoskeletal proteins and their molecular mechanism play an essential role in the proliferation, phenotype modulation and differentiation of PMVECs. However, little is known about the relevance of cytoskeletal protein expression and its molecular mechanism in IPVD of HPS. In addition, ANX A1 protein has been identified as a key regulator in some important signaling pathways, which influences cytoskeletal remodeling in many diseases, such as lung cancer, liver cancer, etc.
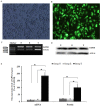
Methods: PMVECs were cultured from the normal rats and then divided into three groups(Ad-ANXA1-transfected group, a non-transfected group, and an adenovirus empty vector group) and incubated by nomal rat serum or hepatopulmonary syndrome rat serum respectively. mRNA level was evaluated by real time reverse transcription polymerase chain reaction, and protein expression was detected by western blot. Cell proliferation was determined by the MTT and thymidine incorporation assay.
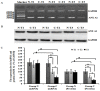
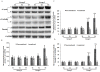
Results: In this study, we found that the serum from a common bile duct ligation(CBDL) Rat model decreased the expression levels of the ANX A1 mRNA and protein by at least two-fold in human PMVECs. We also found the expression of cytoskeletal proteins (Destrin, a1-actin, and a1-tubulin) in PMVECs significantly increased. After stimulating ANX A1 over-expression in PMVECs by adenovirus-mediated ANX A1 (Ad-ANXA1) transfection, we found the expression levels of cytoskeletal proteins were significantly suppressed in PMVECs at all time points. Additionally, we report here that serum from a CBDL Rat model increases the proliferation of PMVECs by nearly two-fold and that over-expression of Ad-ANXA1 significantly inhibits HPS-rat-serum-induced PMVEC proliferation (p <0.05). These findings suggest that the ANX A1 down-regulation of PMVEC proliferation in the presence of HPS-rat-serum may be the major cause of aberrant dysregulation of cytoskeletal proteins (Destrin, a1-actin, and a1-tubulin) and may, therefore, play a fundamental role in the proliferation and phenotype differentiation of PMVECs in the PVD of HPS.
Conclusion: Finally, the fact that transfection with Ad-ANXA1 results in inhibition of the aberrant dysregulation of cytoskeletal proteins and proliferation of PMVECs suggests a potential therapeutic effect on PVD of HPS.
Figures

Similar articles
-
Bone morphogenic protein-2 regulates the myogenic differentiation of PMVECs in CBDL rat serum-induced pulmonary microvascular remodeling.Exp Cell Res. 2015 Aug 1;336(1):109-18. doi: 10.1016/j.yexcr.2015.05.025. Epub 2015 Jun 10. Exp Cell Res. 2015. PMID: 26071935
-
Paxillin suppresses the proliferation of HPS rat serum treated PASMCs by up-regulating the expression of cytoskeletal proteins.Mol Biosyst. 2014 Apr;10(4):759-66. doi: 10.1039/c3mb70391f. Epub 2014 Jan 23. Mol Biosyst. 2014. PMID: 24457422
-
Loss of cell polarity regulated by PTEN/Cdc42 enrolled in the process of Hepatopulmonary Syndrome.J Cell Mol Med. 2019 Aug;23(8):5542-5552. doi: 10.1111/jcmm.14437. Epub 2019 May 29. J Cell Mol Med. 2019. PMID: 31144461 Free PMC article.
-
Hepatopulmonary syndrome: update on pathogenesis and clinical features.Nat Rev Gastroenterol Hepatol. 2012 Sep;9(9):539-49. doi: 10.1038/nrgastro.2012.123. Epub 2012 Jul 3. Nat Rev Gastroenterol Hepatol. 2012. PMID: 22751459 Free PMC article. Review.
-
Endothelial glycocalyx in hepatopulmonary syndrome: An indispensable player mediating vascular changes.Front Immunol. 2022 Dec 22;13:1039618. doi: 10.3389/fimmu.2022.1039618. eCollection 2022. Front Immunol. 2022. PMID: 36618396 Free PMC article. Review.
Cited by
-
Ezrin Regulating the Cytoskeleton Remodeling is Required for Hypoxia-Induced Myofibroblast Proliferation and Migration.Front Cardiovasc Med. 2015 Mar 3;2:10. doi: 10.3389/fcvm.2015.00010. eCollection 2015. Front Cardiovasc Med. 2015. PMID: 26664882 Free PMC article.
-
Potential Clinical Targets in Hepatopulmonary Syndrome: Lessons From Experimental Models.Hepatology. 2018 Nov;68(5):2016-2028. doi: 10.1002/hep.30079. Hepatology. 2018. PMID: 29729196 Free PMC article. Review.
-
Semen Ziziphi Spinosae attenuates blood-brain barrier dysfunction induced by lipopolysaccharide by targeting the FAK-DOCK180-Rac1-WAVE2-Arp3 signaling pathway.NPJ Sci Food. 2022 Jun 2;6(1):27. doi: 10.1038/s41538-022-00142-6. NPJ Sci Food. 2022. PMID: 35655066 Free PMC article.
-
MiR145-5p inhibits proliferation of PMVECs via PAI-1 in experimental hepatopulmonary syndrome rat pulmonary microvascular hyperplasia.Biol Open. 2019 Nov 4;8(11):bio044800. doi: 10.1242/bio.044800. Biol Open. 2019. PMID: 31649116 Free PMC article.
-
Annexin A1/Formyl Peptide Receptor Pathway Controls Uterine Receptivity to the Blastocyst.Cells. 2020 May 11;9(5):1188. doi: 10.3390/cells9051188. Cells. 2020. PMID: 32403233 Free PMC article.
References
-
- Kammoun T, Ben Abdallah R, Chabchoub I, Bahloul S, Aloulou H, Mahfoudh A, Hachicha M. Hepatopulmonary syndrome and portal hypertension. Tunis Med. 2007;85:170–173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

