Ethanol inducible expression of a mesophilic cellulase avoids adverse effects on plant development
- PMID: 23587418
- PMCID: PMC3643885
- DOI: 10.1186/1754-6834-6-53
Ethanol inducible expression of a mesophilic cellulase avoids adverse effects on plant development
Abstract
Background: Plant-produced biomass-degrading enzymes are promising tools for the processing of lignocellulose to fermentable sugars. A major limitation of in planta production is that high-level expression of such enzymes could potentially affect the structure and integrity of the plant cell wall and negatively influence plant growth and development.
Results: Here, we evaluate the impact on tobacco plant development of constitutive versus alcohol-inducible expression of the endoglucanase TrCel5A from the mesophilic fungus Trichoderma reesei. Using this system, we are able to demonstrate that constitutive expression of the enzyme, controlled by the doubled Cauliflower Mosaic Virus promoter, leads to lower cellulose content of the plant combined with severe effects on plant growth. However, using an alcohol-inducible expression of the endoglucanase in the plant leaves, we achieved similar enzymatic expression levels with no changes in the crystalline cellulose content.
Conclusion: We were able to produce significant amounts of cellulase in the plant leaves without detrimental effects to plant development. These results demonstrate the potential feasibility of an inducible expression system for producing biomass degrading enzymes in plants.
Figures


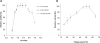
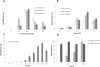


Similar articles
-
Cell wall modification in tobacco by differential targeting of recombinant endoglucanase from Trichoderma reesei.BMC Plant Biol. 2015 Feb 13;15:54. doi: 10.1186/s12870-015-0443-3. BMC Plant Biol. 2015. PMID: 25849300 Free PMC article.
-
Hyperthermophilic endoglucanase for in planta lignocellulose conversion.Biotechnol Biofuels. 2012 Aug 28;5(1):63. doi: 10.1186/1754-6834-5-63. Biotechnol Biofuels. 2012. PMID: 22928996 Free PMC article.
-
Improving the fermentable sugar yields of wheat straw by high-temperature pre-hydrolysis with thermophilic enzymes of Malbranchea cinnamomea.Microb Cell Fact. 2020 Jul 25;19(1):149. doi: 10.1186/s12934-020-01408-y. Microb Cell Fact. 2020. PMID: 32711527 Free PMC article.
-
Deciphering the molecular mechanisms behind cellulase production in Trichoderma reesei, the hyper-cellulolytic filamentous fungus.Biosci Biotechnol Biochem. 2016 Sep;80(9):1712-29. doi: 10.1080/09168451.2016.1171701. Epub 2016 Apr 14. Biosci Biotechnol Biochem. 2016. PMID: 27075508 Review.
-
Heterologous expression of plant cell wall degrading enzymes for effective production of cellulosic biofuels.J Biomed Biotechnol. 2012;2012:405842. doi: 10.1155/2012/405842. Epub 2012 Jul 15. J Biomed Biotechnol. 2012. PMID: 22911272 Free PMC article. Review.
Cited by
-
The TcEG1 beetle (Tribolium castaneum) cellulase produced in transgenic switchgrass is active at alkaline pH and auto-hydrolyzes biomass for increased cellobiose release.Biotechnol Biofuels. 2017 Nov 30;10:230. doi: 10.1186/s13068-017-0918-6. eCollection 2017. Biotechnol Biofuels. 2017. PMID: 29213306 Free PMC article.
-
Cell wall modification in tobacco by differential targeting of recombinant endoglucanase from Trichoderma reesei.BMC Plant Biol. 2015 Feb 13;15:54. doi: 10.1186/s12870-015-0443-3. BMC Plant Biol. 2015. PMID: 25849300 Free PMC article.
-
Comparison of Thermobifida fusca Cellulases Expressed in Escherichia coli and Nicotiana tabacum Indicates Advantages of the Plant System for the Expression of Bacterial Cellulases.Front Plant Sci. 2015 Dec 1;6:1047. doi: 10.3389/fpls.2015.01047. eCollection 2015. Front Plant Sci. 2015. PMID: 26648951 Free PMC article.
-
Expression of recombinant cellulase Cel5A from Trichoderma reesei in tobacco plants.J Vis Exp. 2014 Jun 13;(88):51711. doi: 10.3791/51711. J Vis Exp. 2014. PMID: 24962636 Free PMC article.
-
Strategies for the production of cell wall-deconstructing enzymes in lignocellulosic biomass and their utilization for biofuel production.Plant Biotechnol J. 2016 Jun;14(6):1329-44. doi: 10.1111/pbi.12505. Epub 2015 Dec 2. Plant Biotechnol J. 2016. PMID: 26627868 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

