Kappa-opioid receptor-selective dicarboxylic ester-derived salvinorin A ligands
- PMID: 23587424
- PMCID: PMC3651692
- DOI: 10.1016/j.bmcl.2013.03.111
Kappa-opioid receptor-selective dicarboxylic ester-derived salvinorin A ligands
Abstract
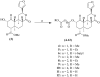
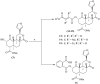
Salvinorin A, the active ingredient of the hallucinogenic plant Salvia divinorum is the most potent known naturally occurring hallucinogen and is a selective κ-opioid receptor agonist. To better understand the ligand-receptor interactions, a series of dicarboxylic ester-type of salvinorin A derivatives were synthesized and evaluated for their binding affinity at κ-, δ- and μ-opioid receptors. Most of the analogues show high affinity to the κ-opioid receptor. Methyl malonyl derivative 4 shows the highest binding affinity (Ki=2nM), analogues 5, 7, and 14 exhibit significant affinity for the κ-receptor (Ki=21, 36 and 39nM).
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
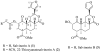
Similar articles
-
Salvinorin A, an active component of the hallucinogenic sage salvia divinorum is a highly efficacious kappa-opioid receptor agonist: structural and functional considerations.J Pharmacol Exp Ther. 2004 Mar;308(3):1197-203. doi: 10.1124/jpet.103.059394. Epub 2004 Jan 8. J Pharmacol Exp Ther. 2004. PMID: 14718611
-
New neoclerodane diterpenoids isolated from the leaves of Salvia divinorum and their binding affinities for human kappa opioid receptors.Bioorg Med Chem. 2005 Oct 1;13(19):5635-9. doi: 10.1016/j.bmc.2005.05.054. Bioorg Med Chem. 2005. PMID: 16084728
-
A unique natural selective kappa-opioid receptor agonist, salvinorin A, and its roles in human therapeutics.Phytochemistry. 2017 May;137:9-14. doi: 10.1016/j.phytochem.2017.02.001. Epub 2017 Feb 10. Phytochemistry. 2017. PMID: 28190678
-
A review of salvinorin analogs and their kappa-opioid receptor activity.Bioorg Med Chem Lett. 2018 May 15;28(9):1436-1445. doi: 10.1016/j.bmcl.2018.03.029. Epub 2018 Mar 12. Bioorg Med Chem Lett. 2018. PMID: 29615341 Free PMC article. Review.
-
Neuropharmacology of the naturally occurring kappa-opioid hallucinogen salvinorin A.Pharmacol Rev. 2011 Jun;63(2):316-47. doi: 10.1124/pr.110.003244. Epub 2011 Mar 28. Pharmacol Rev. 2011. PMID: 21444610 Free PMC article. Review.
Cited by
-
Salvinorin A analogues PR-37 and PR-38 attenuate compound 48/80-induced itch responses in mice.Br J Pharmacol. 2015 Sep;172(17):4331-41. doi: 10.1111/bph.13212. Epub 2015 Jul 14. Br J Pharmacol. 2015. PMID: 26040667 Free PMC article.
-
Michael acceptor approach to the design of new salvinorin A-based high affinity ligands for the kappa-opioid receptor.Eur J Med Chem. 2014 Oct 6;85:818-29. doi: 10.1016/j.ejmech.2014.07.077. Epub 2014 Aug 23. Eur J Med Chem. 2014. PMID: 25193297 Free PMC article.
-
Efficient and markerless gene integration with SlugCas9-HF in Kluyveromyces marxianus.Commun Biol. 2024 Jul 2;7(1):797. doi: 10.1038/s42003-024-06487-w. Commun Biol. 2024. PMID: 38956406 Free PMC article.
-
Addressing Structural Flexibility at the A-Ring on Salvinorin A: Discovery of a Potent Kappa-Opioid Agonist with Enhanced Metabolic Stability.J Med Chem. 2017 May 11;60(9):3866-3878. doi: 10.1021/acs.jmedchem.7b00148. Epub 2017 Apr 19. J Med Chem. 2017. PMID: 28376298 Free PMC article.
-
Cutting-Edge Search for Safer Opioid Pain Relief: Retrospective Review of Salvinorin A and Its Analogs.Front Psychiatry. 2019 Mar 27;10:157. doi: 10.3389/fpsyt.2019.00157. eCollection 2019. Front Psychiatry. 2019. PMID: 30971961 Free PMC article. Review.
References
-
- Ortega A, Blount JF, Manchand PS. J. Chem. Soc., Perkin Trans.1. 1982:2505–2508.
-
- Beguin C, Potuzak J, Xu W, Liu - Chen LY, Streicher JM, Groer CE, Bohn LM, Carlezon WA, Jr., Cohen BM. Bioorg. Med. Chem. Lett. 2012;22:1023–1026. - PMC - PubMed
- Lovell KM, Vasiljevik T, Araya JJ, Lozama A, Prevatt-Smith KM, Day VW, Dersch CM, Rothman RB, Butelman ER, Kreek MJ, Prisinzano TE. Bioorg. Med. Chem. 2012;20:3100–3110. - PMC - PubMed
- Cunningham CW, Rothman RB, Prisinzano TE. Pharmacol. Rev. 2011;63:316–347. - PMC - PubMed
- Lozama A, Cunningham CW, Caspers MJ, Douglas JT, Dersch CM, Rothman RB, Prisinzano TE. J. Nat. Prod. 2011;74:718–726. - PMC - PubMed
- Tidgewell K, Harding WW, Lozama A, Cobb H, Shah K, Kannan P, Dersch CM, Parrish D, Deschamps JR, Rothman RB, Prisinzano TE. J. Nat. Prod. 2006;69:914–918. - PubMed
- Bikbulatov RV, Yan F, Roth BL, Zjawiony JK. Bioorg. Med. Chem. Lett. 2007;17:2229–2232. - PMC - PubMed
- Lee DY, He M, Liu-Chen L-Y, Wang Y, Li J-G, Xu W, Ma Z, Carlezon WA, Jr., Cohen B. Bioorg. Med. Chem. Lett. 2006;16:5498–5502. - PubMed
- Stewart DJ, Fahmy H, Roth BL, Yan F, Zjawiony JK. Arzneimittelforschung. 2006;56:269–275. - PubMed
- Beguin C, Richards MR, Li JG, Wang Y, Xu W, Liu - Chen LY, Carlezon WA, Jr., Cohen BM. Bioorg. Med. Chem. Lett. 2006;16:4679–4685. - PubMed
- Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, Prisinzano TE. J. Med. Chem. 2005;48:4765–4771. - PubMed
- Lee DY, Yang L, Xu W, Deng G, Guo L, Liu-Chen LY. Bioorg. Med. Chem. Lett. 2010;20:5749–5752. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

