Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial
- PMID: 23587561
- PMCID: PMC3636323
- DOI: 10.2196/jmir.2283
Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial
Abstract
Background: There is growing interest in the use of information communication technologies to treat obesity. An intervention delivered by smartphone could be a convenient, potentially cost-effective, and wide-reaching weight management strategy. Although there have been studies of texting-based interventions and smartphone applications (apps) used as adjuncts to other treatments, there are currently no randomized controlled trials (RCT) of a stand-alone smartphone application for weight loss that focuses primarily on self-monitoring of diet and physical activity.
Objective: The aim of this pilot study was to collect acceptability and feasibility outcomes of a self-monitoring weight management intervention delivered by a smartphone app, compared to a website and paper diary.
Methods: A sample of 128 overweight volunteers were randomized to receive a weight management intervention delivered by smartphone app, website, or paper diary. The smartphone app intervention, My Meal Mate (MMM), was developed by the research team using an evidence-based behavioral approach. The app incorporates goal setting, self-monitoring of diet and activity, and feedback via weekly text message. The website group used an existing commercially available slimming website from a company called Weight Loss Resources who also provided the paper diaries. The comparator groups delivered a similar self-monitoring intervention to the app, but by different modes of delivery. Participants were recruited by email, intranet, newsletters, and posters from large local employers. Trial duration was 6 months. The intervention and comparator groups were self-directed with no ongoing human input from the research team. The only face-to-face components were at baseline enrollment and brief follow-up sessions at 6 weeks and 6 months to take anthropometric measures and administer questionnaires.
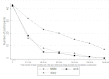
Results: Trial retention was 40/43 (93%) in the smartphone group, 19/42 (55%) in the website group, and 20/43 (53%) in the diary group at 6 months. Adherence was statistically significantly higher in the smartphone group with a mean of 92 days (SD 67) of dietary recording compared with 35 days (SD 44) in the website group and 29 days (SD 39) in the diary group (P<.001). Self-monitoring declined over time in all groups. In an intention-to-treat analysis using baseline observation carried forward for missing data, mean weight change at 6 months was -4.6 kg (95% CI -6.2 to -3.0) in the smartphone app group, -2.9 kg (95% CI -4.7 to -1.1) in the diary group, and -1.3 kg (95% CI -2.7 to 0.1) in the website group. BMI change at 6 months was -1.6 kg/m(2) (95% CI -2.2 to -1.1) in the smartphone group, -1.0 kg/m(2) (95% CI -1.6 to -0.4) in the diary group, and -0.5 kg/m(2) (95% CI -0.9 to 0.0) in the website group. Change in body fat was -1.3% (95% CI -1.7 to -0.8) in the smartphone group, -0.9% (95% CI -1.5 to -0.4) in the diary group, and -0.5% (95% CI -0.9 to 0.0) in the website group.
Conclusions: The MMM app is an acceptable and feasible weight loss intervention and a full RCT of this approach is warranted.
Trial registration: ClinicalTrials.gov NCT01744535; http://clinicaltrials.gov/ct2/show/NCT01744535 (Archived by WebCite at http://www.webcitation.org/6FEtc3PVB).
Conflict of interest statement
Conflicts of Interest: The authors developed the My Meal Mate (MMM) application for the purposes of this trial by working with a software company (Blueberry Consultants). The University of Leeds owns full intellectual property of the MMM app. The researchers developed the application and objectively evaluated it, but have no commercial intent with the app, which is available for free download.
Figures


References
-
- World Health Organization. [2012-07-24]. Global database on body mass index http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
-
- The Information Centre for Health and Social Care. 2012. Feb 23, [2012-07-25]. Statistics on Obesity, Physical Activity and Diet: England, 2012 http://www.ic.nhs.uk/pubs/opad12.
-
- Butland B, Jebb S, Kopelman P, McPherson K, Thomas S, Mardell J. Foresight Tackling Obesities: Future Choices-Project Report. UK: Government Office for Science; 2007. [2013-02-08]. http://www.bis.gov.uk/assets/foresight/docs/obesity/17.pdf.
-
- Jolly K, Lewis A, Beach J, Denley J, Adab P, Deeks JJ, Daley A, Aveyard P. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: lighten Up randomised controlled trial. BMJ. 2011;343:d6500. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=22053315 - PMC - PubMed
-
- Sherwood NE, Morton N, Jeffery RW, French SA, Neumark-Sztainer D, Falkner NH. Consumer preferences in format and type of community-based weight control programs. Am J Health Promot. 1998;13(1):12–8. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

