Identification of a small molecule that induces ATG5-and-cathepsin-l-dependent cell death and modulates polyglutamine toxicity
- PMID: 23588206
- PMCID: PMC3700633
- DOI: 10.1016/j.yexcr.2013.03.019
Identification of a small molecule that induces ATG5-and-cathepsin-l-dependent cell death and modulates polyglutamine toxicity
Abstract
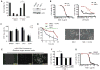
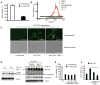
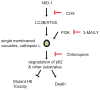
Non-apoptotic cell death mechanisms are largely uncharacterized despite their importance in physiology and disease [1]. Here we sought to systematically identify non-apoptotic cell death pathways in mammalian cells. We screened 69,612 compounds for those that induce non-canonical cell death by counter screening in the presence of inhibitors of apoptosis and necrosis. We further selected compounds that require active protein synthesis for inducing cell death. Using this tiered approach, we identified NID-1 (Novel Inducer of Death-1), a small molecule that induces an active, energy-dependent cell death in diverse mammalian cell lines. NID-1-induced death required components of the autophagic machinery, including ATG5, and the lysosomal hydrolase cathepsin L, but was distinct from classical macroautophagy. Since macroautophagy can prevent cell death in several contexts, we tested and found that NID-1 suppressed cell death in a cell-based model of Huntington's disease, suggesting that NID-1 activates a specific pathway. Thus the discovery of NID-1 identifies a previously unexplored cell death pathway, and modulating this pathway may have therapeutic applications. Furthermore, these findings provide a proof-of-principle for using chemical screening to identify novel cell death paradigms.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





References
-
- Kreuzaler P, Watson CJ. Killing a cancer: what are the alternatives? Nat Rev Cancer. 12:411–424. - PubMed
-
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. - PubMed
-
- Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

