Replication of TPMT and ABCC3 genetic variants highly associated with cisplatin-induced hearing loss in children
- PMID: 23588304
- PMCID: PMC4006820
- DOI: 10.1038/clpt.2013.80
Replication of TPMT and ABCC3 genetic variants highly associated with cisplatin-induced hearing loss in children
Abstract
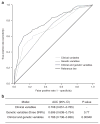
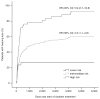
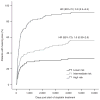
Cisplatin is a widely used chemotherapeutic agent for the treatment of solid tumors. A serious complication of cisplatin treatment is permanent hearing loss. The aim of this study was to replicate previous genetic findings in an independent cohort of 155 pediatric patients. Associations were replicated for genetic variants in TPMT (rs12201199, P = 0.0013, odds ratio (OR) 6.1) and ABCC3 (rs1051640, P = 0.036, OR 1.8). A predictive model combining variants in TPMT, ABCC3, and COMT with clinical variables (patient age, vincristine treatment, germ-cell tumor, and cranial irradiation) significantly improved the prediction of hearing-loss development as compared with using clinical risk factors alone (area under the curve (AUC) 0.786 vs. 0.708, P = 0.00048). The novel combination of genetic and clinical factors predicted the risk of hearing loss with a sensitivity of 50.3% and a specificity of 92.7%. These findings provide evidence to support the importance of TPMT, COMT, and ABCC3 in the prediction of cisplatin-induced hearing loss in children.
Conflict of interest statement
The authors declared no conflict of interest.
Figures
Comment in
-
Genetics of cisplatin ototoxicity: confirming the unexplained?Clin Pharmacol Ther. 2013 Aug;94(2):198-200. doi: 10.1038/clpt.2013.116. Clin Pharmacol Ther. 2013. PMID: 23872836
-
Challenges in interpreting the evidence for genetic predictors of ototoxicity.Clin Pharmacol Ther. 2013 Dec;94(6):631-5. doi: 10.1038/clpt.2013.178. Clin Pharmacol Ther. 2013. PMID: 24241639
-
Evaluation of pharmacogenetic markers to predict the risk of Cisplatin-induced ototoxicity.Clin Pharmacol Ther. 2014 Aug;96(2):156-7. doi: 10.1038/clpt.2014.67. Epub 2014 Mar 18. Clin Pharmacol Ther. 2014. PMID: 24642735 No abstract available.
-
Response to "evaluation of pharmacogenetic markers to predict the risk of Cisplatin-induced ototoxicity".Clin Pharmacol Ther. 2014 Aug;96(2):158. doi: 10.1038/clpt.2014.90. Epub 2014 Apr 22. Clin Pharmacol Ther. 2014. PMID: 24755913 No abstract available.
References
-
- Perilongo G, et al. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med. 2009;361:1662–1670. - PubMed
-
- Kling J. US FDA contemplates collection of pharmacogenomic data. Nat Biotechnol. 2003;21:590. - PubMed
-
- Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. - PubMed
-
- Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol. 1991;19:295–300. - PubMed
-
- Blakley BW, Myers SF. Patterns of hearing loss resulting from cis-platinum therapy. Otolaryngol Head Neck Surg. 1993;109:385–391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

