Biomarkers for smoking cessation
- PMID: 23588313
- PMCID: PMC3772534
- DOI: 10.1038/clpt.2013.57
Biomarkers for smoking cessation
Abstract
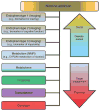
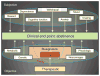
One way to enhance therapeutic development is through the identification and development of evaluative tools such as biomarkers. This review focuses on putative diagnostic, pharmacodynamic, and predictive biomarkers for smoking cessation. These types of biomarkers may be used to more accurately diagnose a disease, personalize treatment, identify novel targets for drug discovery, and enhance the efficiency of drug development. Promising biomarkers are presented across a range of approaches including metabolism, genetics, and neuroimaging. A preclinical viewpoint is also offered, as are analytical considerations and a regulatory perspective summarizing a pathway toward biomarker qualification.
Conflict of interest statement
C.L. has received funding and/or served as a consultant to GlaxoSmithKline, Pfizer, and AstraZeneca. R.F.T. has participated in 1-day advisory meetings for Novartis and McNeil. Associate Editor R.F.T. was not involved in the review or decision process for this article. F.J.M. has received research funding from Pfizer (primary investigator: Munafo). J.E.R. and G.R.U. are listed as co-inventors on a patent application filed by Duke University based on genomic markers that distinguish successful quitters from unsuccessful quitters. S.P.D. has consulted for a 1-day Pfizer-sponsored conference on behavioral treatments for smoking cessation and is a scientific advisor to Genophen. D.V.C. has served as a consultant to Pfizer. The other authors declared no conflict of interest.
Figures


References
-
- World Health Organization. < http://www.who.int/tobacco/enpower/tobacco_facts/en/>.
-
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–1228. - PubMed
-
- Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report (MMWR) < http://www.cdc.gov/mmwr/preview/mmwrhtml/SH6004a12/htm>.
-
- Centers for Disease Control and Prevention. National Health Interview Survey, United States. 2005–2010 < http://www.cdc.gov/mmwr/pdf/wk/mm6035.pdf#page=21>.
-
- Bauld L, Bell K, McCullough L, Richardson L, Greaves L. The effectiveness of NHS smoking cessation services: a systematic review. J Public Health (Oxf) 2010;32:71–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA084718/CA/NCI NIH HHS/United States
- R01DA025983/DA/NIDA NIH HHS/United States
- P50 DA027840/DA/NIDA NIH HHS/United States
- P50 CA143187/CA/NCI NIH HHS/United States
- R01 ES019876/ES/NIEHS NIH HHS/United States
- R01 DA025876/DA/NIDA NIH HHS/United States
- TMH-109787/CAPMC/ CIHR/Canada
- U01 DA020830/DA/NIDA NIH HHS/United States
- P50 DA009262/DA/NIDA NIH HHS/United States
- R01 CA140561/CA/NCI NIH HHS/United States
- MOP-86471/CAPMC/ CIHR/Canada
- R21DA027331/DA/NIDA NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01 DA017441/DA/NIDA NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- R21 DA027331/DA/NIDA NIH HHS/United States
- R01 DA025983/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

