Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis
- PMID: 23588595
- PMCID: PMC3647098
- DOI: 10.1007/s40265-013-0041-3
Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis
Abstract
Background: Non-adherence to medications is prevalent across all medical conditions that include ambulatory pharmacotherapy and is thus a major barrier to achieving the benefits of otherwise effective medicines.
Objective: The objective of this systematic review was to identify and to compare the efficacy of strategies and components thereof that improve implementation of the prescribed drug dosing regimen and maintain long-term persistence, based on quantitative evaluation of effect sizes across the aggregated trials.
Data sources: MEDLINE, EMBASE, CINAHL, the Cochrane Library, and PsycINFO were systematically searched for randomized controlled trials that tested the efficacy of adherence-enhancing strategies with self-administered medications. The searches were limited to papers in the English language and were included from database inception to 31 December 2011.
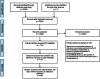
Study selection: Our review included randomized controlled trials in which adherence was assessed by electronically compiled drug dosing histories. Five thousand four hundred studies were screened. Eligibility assessment was performed independently by two reviewers. A structured data collection sheet was developed to extract data from each study.
Study appraisal and synthesis methods: The adherence-enhancing components were classified in eight categories. Quality of the papers was assessed using the criteria of the Cochrane Handbook for Systematic Reviews of Interventions guidelines to assess potential bias. A combined adherence outcome was derived from the different adherence variables available in the studies by extracting from each paper the available adherence summary variables in a pre-defined order (correct dosing, taking adherence, timing adherence, percentage of adherent patients). To study the association between the adherence-enhancing components and their effect on adherence, a linear meta-regression model, based on mean adherence point estimates, and a meta-analysis were conducted.
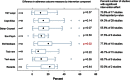
Results: Seventy-nine clinical trials published between 1995 and December 2011 were included in the review. Patients randomized to an intervention group had an average combined adherence outcome of 74.3 %, which was 14.1 % higher than in patients randomized to the control group (60.2 %). The linear meta-regression analysis with stepwise variable selection estimated an 8.8 % increase in adherence when the intervention included feedback to the patients of their recent dosing history (EM-feedback) (p < 0.01) and a 5.0 % increase in adherence when the intervention included a cognitive-educational component (p = 0.02). In addition, the effect of interventions on adherence decreased by 1.1 % each month. Sensitivity analysis by selecting only high-quality papers confirmed the robustness of the model. The random effects model in the meta-analysis, conducted on 48 studies, confirmed the above findings and showed that the improvement in adherence was 19.8 % (95 % CI 10.7-28.9 %) among patients receiving EM-feedback, almost double the improvement in adherence for studies that did not include this type of feedback [10.3 % (95 % CI 7.5-13.1 %)] (p < 0.01). The improvement in adherence was 16.1 % (95 % CI 10.7-21.6 %) in studies that tested cognitive-educational components versus 10.1 % (95 % CI 6.6-13.6 %) in studies that did not include this type of intervention (p = 0.04). Among 57 studies measuring clinical outcomes, only 8 reported a significant improvement in clinical outcome.
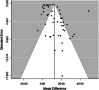
Limitations: Despite a common measurement, the meta-analysis was limited by the heterogeneity of the pooled data and the different measures of medication adherence. The funnel plot showed a possible publication bias in studies with high variability of the intervention effect.
Conclusions: Notwithstanding the statistical heterogeneity among the studies identified, and potential publication bias, the evidence from our meta-analysis suggests that EM-feedback and cognitive-educational interventions are potentially effective approaches to enhance patient adherence to medications. The limitations of this research highlight the urgent need to define guidelines and study characteristics for research protocols that can guide researchers in designing studies to assess the effects of adherence-enhancing interventions.
Figures



Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
The effectiveness of therapeutic patient education on adherence to oral anti-cancer medicines in adult cancer patients in ambulatory care settings: a systematic review.JBI Database System Rev Implement Rep. 2015 Jun 12;13(5):244-92. doi: 10.11124/jbisrir-2015-2057. JBI Database System Rev Implement Rep. 2015. PMID: 26455611
-
Shared decision-making for people with asthma.Cochrane Database Syst Rev. 2017 Oct 3;10(10):CD012330. doi: 10.1002/14651858.CD012330.pub2. Cochrane Database Syst Rev. 2017. PMID: 28972652 Free PMC article.
-
Interventions for promoting habitual exercise in people living with and beyond cancer.Cochrane Database Syst Rev. 2018 Sep 19;9(9):CD010192. doi: 10.1002/14651858.CD010192.pub3. Cochrane Database Syst Rev. 2018. PMID: 30229557 Free PMC article.
-
Interventions for patients and caregivers to improve knowledge of sickle cell disease and recognition of its related complications.Cochrane Database Syst Rev. 2016 Oct 6;10(10):CD011175. doi: 10.1002/14651858.CD011175.pub2. Cochrane Database Syst Rev. 2016. PMID: 27711980 Free PMC article.
Cited by
-
Electronic Monitoring Feedback for Improving Medication Adherence and Clinical Outcomes in Early Rheumatoid Arthritis: A Randomized Clinical Trial.Patient Prefer Adherence. 2021 May 25;15:1107-1119. doi: 10.2147/PPA.S297170. eCollection 2021. Patient Prefer Adherence. 2021. PMID: 34079231 Free PMC article.
-
Electronic medication monitoring-informed counseling to improve adherence to combination anti-retroviral therapy and virologic treatment outcomes: a meta-analysis.Front Public Health. 2015 May 19;3:139. doi: 10.3389/fpubh.2015.00139. eCollection 2015. Front Public Health. 2015. PMID: 26042212 Free PMC article.
-
Adherence of Geriatric Patients and Their Beliefs toward Their Medicines in the United Arab Emirates.J Pharm Bioallied Sci. 2020 Jan-Mar;12(1):22-30. doi: 10.4103/jpbs.JPBS_93_19. Epub 2020 Jan 29. J Pharm Bioallied Sci. 2020. PMID: 32801597 Free PMC article.
-
Adherence to Antibiotic Prescription of Dental Patients: The Other Side of the Antimicrobial Resistance.Healthcare (Basel). 2022 Aug 27;10(9):1636. doi: 10.3390/healthcare10091636. Healthcare (Basel). 2022. PMID: 36141247 Free PMC article.
-
Why it Worked: Participants' Insights into an mHealth Antiretroviral Therapy Adherence Intervention in China.Open AIDS J. 2018 Mar 12;12:20-37. doi: 10.2174/1874613601812010020. eCollection 2018. Open AIDS J. 2018. PMID: 29576816 Free PMC article.
References
-
- Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

