An autoinhibited structure of α-catenin and its implications for vinculin recruitment to adherens junctions
- PMID: 23589308
- PMCID: PMC3668747
- DOI: 10.1074/jbc.M113.453928
An autoinhibited structure of α-catenin and its implications for vinculin recruitment to adherens junctions
Abstract
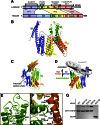
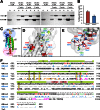
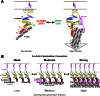
α-Catenin is an actin- and vinculin-binding protein that regulates cell-cell adhesion by interacting with cadherin adhesion receptors through β-catenin, but the mechanisms by which it anchors the cadherin-catenin complex to the actin cytoskeleton at adherens junctions remain unclear. Here we determined crystal structures of αE-catenin in the autoinhibited state and the actin-binding domain of αN-catenin. Together with the small-angle x-ray scattering analysis of full-length αN-catenin, we deduced an elongated multidomain assembly of monomeric α-catenin that structurally and functionally couples the vinculin- and actin-binding mechanisms. Cellular and biochemical studies of αE- and αN-catenins show that αE-catenin recruits vinculin to adherens junctions more effectively than αN-catenin, partly because of its higher affinity for actin filaments. We propose a molecular switch mechanism involving multistate conformational changes of α-catenin. This would be driven by actomyosin-generated tension to dynamically regulate the vinculin-assisted linkage between adherens junctions and the actin cytoskeleton.
Keywords: Adherens Junction; Cadherins; Catenin; Cell Adhesion; Cytoskeleton; Vinculin; α-Catenin.
Figures




References
-
- Vasioukhin V., Fuchs E. (2001) Actin dynamics and cell-cell adhesion in epithelia. Curr. Opin. Cell Biol. 13, 76–84 - PubMed
-
- Takeichi M. (1995) Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 7, 619–627 - PubMed
-
- Ishiyama N., Lee S.-H., Liu S., Li G.-Y., Smith M. J., Reichardt L. F., Ikura M. (2010) Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell 141, 117–128 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

