Crystal and solution studies reveal that the transcriptional regulator AcnR of Corynebacterium glutamicum is regulated by citrate-Mg2+ binding to a non-canonical pocket
- PMID: 23589369
- PMCID: PMC3668737
- DOI: 10.1074/jbc.M113.462440
Crystal and solution studies reveal that the transcriptional regulator AcnR of Corynebacterium glutamicum is regulated by citrate-Mg2+ binding to a non-canonical pocket
Abstract
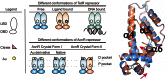
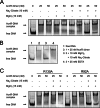
Corynebacterium glutamicum is an important industrial bacterium as well as a model organism for the order Corynebacteriales, whose citric acid cycle occupies a central position in energy and precursor supply. Expression of aconitase, which isomerizes citrate into isocitrate, is controlled by several transcriptional regulators, including the dimeric aconitase repressor AcnR, assigned by sequence identity to the TetR family. We report the structures of AcnR in two crystal forms together with ligand binding experiments and in vivo studies. First, there is a citrate-Mg(2+) moiety bound in both forms, not in the canonical TetR ligand binding site but rather in a second pocket more distant from the DNA binding domain. Second, the citrate-Mg(2+) binds with a KD of 6 mM, within the range of physiological significance. Third, citrate-Mg(2+) lowers the affinity of AcnR for its target DNA in vitro. Fourth, analyses of several AcnR point mutations provide evidence for the possible involvement of the corresponding residues in ligand binding, DNA binding, and signal transfer. AcnR derivatives defective in citrate-Mg(2+) binding severely inhibit growth of C. glutamicum on citrate. Finally, the structures do have a pocket corresponding to the canonical tetracycline site, and although we have not identified a ligand that binds there, comparison of the two crystal forms suggests differences in the region of the canonical pocket that may indicate a biological significance.
Keywords: Corynebacteriales; Crystal Structure; Gene Regulation; Ligand-binding Protein; Metabolic Regulation; Structural Biology; Transcription Regulation.
Figures

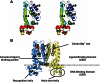





Similar articles
-
Identification of AcnR, a TetR-type repressor of the aconitase gene acn in Corynebacterium glutamicum.J Biol Chem. 2005 Jan 7;280(1):585-95. doi: 10.1074/jbc.M408271200. Epub 2004 Oct 19. J Biol Chem. 2005. PMID: 15494411
-
The crystal structures of apo and cAMP-bound GlxR from Corynebacterium glutamicum reveal structural and dynamic changes upon cAMP binding in CRP/FNR family transcription factors.PLoS One. 2014 Dec 3;9(12):e113265. doi: 10.1371/journal.pone.0113265. eCollection 2014. PLoS One. 2014. PMID: 25469635 Free PMC article.
-
Structure of AmtR, the global nitrogen regulator of Corynebacterium glutamicum, in free and DNA-bound forms.FEBS J. 2016 Mar;283(6):1039-59. doi: 10.1111/febs.13643. Epub 2016 Feb 10. FEBS J. 2016. PMID: 26744254
-
The RamA regulon: complex regulatory interactions in relation to central metabolism in Corynebacterium glutamicum.Appl Microbiol Biotechnol. 2018 Jul;102(14):5901-5910. doi: 10.1007/s00253-018-9085-3. Epub 2018 May 26. Appl Microbiol Biotechnol. 2018. PMID: 29804137 Review.
-
Transcriptional regulators of multiple genes involved in carbon metabolism in Corynebacterium glutamicum.J Biotechnol. 2011 Jul 10;154(2-3):114-25. doi: 10.1016/j.jbiotec.2011.01.016. Epub 2011 Jan 26. J Biotechnol. 2011. PMID: 21277916 Review.
Cited by
-
IpsA, a novel LacI-type regulator, is required for inositol-derived lipid formation in Corynebacteria and Mycobacteria.BMC Biol. 2013 Dec 30;11:122. doi: 10.1186/1741-7007-11-122. BMC Biol. 2013. PMID: 24377418 Free PMC article.
-
The conserved actinobacterial transcriptional regulator FtsR controls expression of ftsZ and further target genes and influences growth and cell division in Corynebacterium glutamicum.BMC Microbiol. 2019 Aug 5;19(1):179. doi: 10.1186/s12866-019-1553-0. BMC Microbiol. 2019. PMID: 31382874 Free PMC article.
-
The TetR family of regulators.Microbiol Mol Biol Rev. 2013 Sep;77(3):440-75. doi: 10.1128/MMBR.00018-13. Microbiol Mol Biol Rev. 2013. PMID: 24006471 Free PMC article. Review.
-
A Versatile Transcription Factor Biosensor System Responsive to Multiple Aromatic and Indole Inducers.ACS Synth Biol. 2022 Apr 15;11(4):1692-1698. doi: 10.1021/acssynbio.2c00063. Epub 2022 Mar 22. ACS Synth Biol. 2022. PMID: 35316041 Free PMC article.
-
The role of the transcriptional repressor CssR in Corynebacterium glutamicum in response to phenolic compounds.Heliyon. 2024 Mar 10;10(6):e27929. doi: 10.1016/j.heliyon.2024.e27929. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38509974 Free PMC article.
References
-
- Kinoshita S., Udaka S., Shimono M. (1957) Studies of amino acid fermentation. I. Production of l-glutamic acid by various microorganisms. J. Gen. Appl. Microbiol. 3, 193–205 - PubMed
-
- Leuchtenberger W., Huthmacher K., Drauz K. (2005) Biotechnological production of amino acids and derivatives. Current status and prospects. Appl. Microbiol. Biotechnol. 69, 1–8 - PubMed
-
- Eggeling L., Bott M. (2005) Handbook of Corynebacterium glutamicum, CRC Press, Inc., Boca Raton, FL
-
- Burkovski A. (2008) Corynebacteria: Genomics and Molecular Biology, Caister Academic Press, Norfolk, UK
-
- Yukawa H., Inui M. (2013) Corynebacterium glutamicum: Biology and Biotechnology, Springer Verlag, Heidelberg
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

