Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase
- PMID: 23589493
- PMCID: PMC3628507
- DOI: 10.1083/jcb.201207139
Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase
Abstract
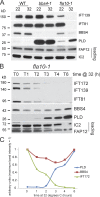
The BBSome is a complex of seven proteins, including BBS4, that is cycled through cilia by intraflagellar transport (IFT). Previous work has shown that the membrane-associated signaling protein phospholipase D (PLD) accumulates abnormally in cilia of Chlamydomonas reinhardtii bbs mutants. Here we show that PLD is a component of wild-type cilia but is enriched ∼150-fold in bbs4 cilia; this accumulation occurs progressively over time and results in altered ciliary lipid composition. When wild-type BBSomes were introduced into bbs cells, PLD was rapidly removed from the mutant cilia, indicating the presence of an efficient BBSome-dependent mechanism for exporting ciliary PLD. This export requires retrograde IFT. Importantly, entry of PLD into cilia is BBSome and IFT independent. Therefore, the BBSome is required only for the export phase of a process that continuously cycles PLD through cilia. Another protein, carbonic anhydrase 6, is initially imported normally into bbs4 cilia but lost with time, suggesting that its loss is a secondary effect of BBSome deficiency.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

