Advancing precision medicine for prostate cancer through genomics
- PMID: 23589550
- PMCID: PMC3808235
- DOI: 10.1200/JCO.2012.45.3662
Advancing precision medicine for prostate cancer through genomics
Abstract
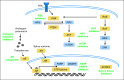
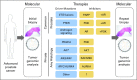
Prostate cancer is the most common type of cancer in men and the second leading cause of cancer death in men in the United States. The recent surge of high-throughput sequencing of cancer genomes has supported an expanding molecular classification of prostate cancer. Translation of these basic science studies into clinically valuable biomarkers for diagnosis and prognosis and biomarkers that are predictive for therapy is critical to the development of precision medicine in prostate cancer. We review potential applications aimed at improving screening specificity in prostate cancer and differentiating aggressive versus indolent prostate cancers. Furthermore, we review predictive biomarker candidates involving ETS gene rearrangements, PTEN inactivation, and androgen receptor signaling. These and other putative biomarkers may signify aberrant oncogene pathway activation and provide a rationale for matching patients with molecularly targeted therapies in clinical trials. Lastly, we advocate innovations for clinical trial design to incorporate tumor biopsy and molecular characterization to develop biomarkers and understand mechanisms of resistance.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. - PubMed
-
- Attard G, de Bono JS. Translating scientific advancement into clinical benefit for castration-resistant prostate cancer patients. Clin Cancer Res. 2011;17:3867–3875. - PubMed
-
- Young RC. Cancer clinical trials: A chronic but curable crisis. N Engl J Med. 2010;363:306–309. - PubMed
-
- Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

